
�ش��������⣺
(1)д����ĭ����������ԭ�������ӷ���ʽ
(2)��֪25�棬101 kPaʱ��
S(s)��O2(g)==SO2(g) ∆H��-296.8 kJ/mol
2Cu(s)��O2(g)==2CuO(s) ∆H��-314.6 kJ/mol
Cu(s)��S(s)==CuS(s) ∆H��-53.1 kJ/mol
д��CuS(s)��O2(g)��Ӧ����CuO(s)��SO2(g)���Ȼ�ѧ����ʽ ��
(3)��֪������AI(OH)3��Ksp=l.0��10-33������Һ��c(Al3+)Ϊ1.0 mol/L�������Al3+��ʼ������pH= �������£�Ũ�Ⱦ�Ϊ0.01mol/L��CH3COOH��CH3COONa�Ļ��Һ�У�PH = a�������ĵ��볣��ԼΪKa = ��
(4)�ڳ����£���V L pH=12��Ba(OH)2��Һ����μ���һ��Ũ�ȵ�NaHSO4ϡ��Һ������Һ�е�Ba2+ ǡ�ó�����ȫʱ����ҺpH=11����Ba(OH)2��Һ��NaHSO4��Һ�������Ϊ ��NaHSO4��Һ�����ʵ���Ũ��Ϊ mol/L��
 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016�������������ʡ��ʡ��У������ѧ������������ѧ�Ծ��������棩 ���ͣ�ѡ����
��ѧ���������ճ�������������Ҫ�����á������й�˵����ȷ���ǣ� ��
A. Ϊ��ֹ�����±��ȸ�֬ʳƷ�����������ʣ����ڰ�װ���з�����ʯ�һ�轺
B. ���������ܽ���ˮ������������Һ��PM2.5�������ж����ЧӦ
C. ������ڼ�ȼ�ŵ������ijЩ����Ԫ����ɫ��Ӧ�����ֳ�����ɫ��
D. Ũ����ɿ�ʴʯӢ������Ʒ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������б������߶�11��������ѧ�Ծ��������棩 ���ͣ������
��֪���з�Ӧ�ķ�Ӧ��Ϊ��
��1��CH3COOH(l)+2O2(g)=2CO2(g)+2H2O(l) ��H1 = -870.3KJ/mol
��2��C(s)+O2(g)=CO2(g) ��H2 = -393.5KJ/mol
��3��H2(g)+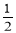 O2(g)=H2O(l) ��H3 = -285.8KJ/mol
O2(g)=H2O(l) ��H3 = -285.8KJ/mol
�Լ������з�Ӧ�ķ�Ӧ�ȣ�
2C(s)+2H2(g)+O2(g)=CH3COOH(l) ?H ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������б������߶�11��������ѧ�Ծ��������棩 ���ͣ�ѡ����
������ʵ��������������ԭ�����͵���( )
A������ơ��ƿ��ƿ�����Ϸ��������ĭ
B�������ڳ�ʪ�Ŀ�������������
C����ҵ�ϳɰ�ʱ����ͨ���������ķ������������ת����
D����ҵ����������Ĺ�����ʹ�ù����Ŀ�������߶��������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������б������߶�11��������ѧ�Ծ��������棩 ���ͣ�ѡ����
���и����Ȼ�ѧ����ʽ�У���ѧ��Ӧ�ġ�Hǰ�ߴ��ں��ߵ���( )
�� C(s)�� O2(g)=CO2(g)����H1 C(s)��O2(g)=CO(g)����H2
O2(g)=CO2(g)����H1 C(s)��O2(g)=CO(g)����H2
�� S(s)��O2(g)=SO2(g)����H3 S(g)��O2(g)=SO2(g)����H4
�� H2(g)��1/2O2(g)==H2O(l)����H5 2H2(g)��O2(g)=2H2O(l)����H6
�� CaCO3(s)=C aO(s)��CO2(g)��H7 CaO(s)��H2O(l)=Ca(OH)(s)����H8
aO(s)��CO2(g)��H7 CaO(s)��H2O(l)=Ca(OH)(s)����H8
A���� B���� C���ڢۢ� D���٢ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���Ĵ�ʡ�߶�12���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
ij��Ӧ���������仯��ͼ��ʾ������˵����ȷ����( )
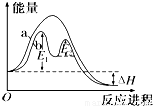
A���ı�������ɸı�÷�Ӧ�Ļ��
B���÷�ӦΪ���ȷ�Ӧ����ЧӦ���ڡ�H
C����Ӧ����a�д�������
D���д��������£���Ӧ�Ļ�ܵ���E1+E2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����¿��Դ����Ӧ�Կ��Ի�ѧ���������棩 ���ͣ�ʵ����
ij��ѧ��ȤС����ʵ����ͨ������ͼ��ʾװ���Ʊ�Na2S2O3��
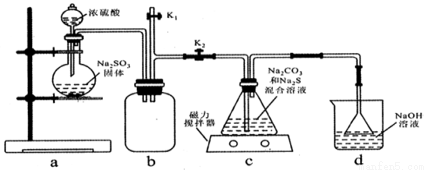
��1��װ��a��ʢװNa2SO3���������������________��
��2��װ��b��������_______��
��3��ʵ�������,װ��d�е�������NaOH��Na2CO3����������________(�ѧʽ)��
��4��װ��c�з�Ӧ�Ļ�ѧ����ʽΪ_____________��
��5��ʵ�������װ��c����Һ��������Ҫ��Na2S2O3��������Na2CO3��Na2SO3�ȳɷݡ�Ϊ��֤��Na2CO3�Ĵ��ڣ��������ʵ�顣��ѡ�Լ�:AƷ����Һ��B���Ը��������Һ��C BaCl2��Һ��D����ʯ��ˮ��E ϡ����
�� ȡC����Һ�������μ�����___________(���Լ���ţ���
�� ���ٲ�������������ͨ��________(���Լ���š���ʵ������ͽ���Ϊ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ӱ�ʡ�����ϵ����νβ⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵����ȷ����
A������ ����ϩ����ȩ������������ͬ���칹����Ŀ���ٵ�����ϩ
B������ʽΪ C5H12O �ҿ�������Ʒ�Ӧ�ų��������л��������� 8 ��
C���� 2�������� NaOH �Ĵ���Һ���ȿ��Ʊ� CH3��CH��CH2
D��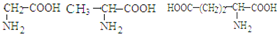 ���ְ�������ˮ���������� 6 �ֶ���
���ְ�������ˮ���������� 6 �ֶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�߶����¿��廯ѧ���������棩 ���ͣ�ѡ����
��ԭ��Ľṹ��ʽ��ͼ�������й���ԭ���˵���������
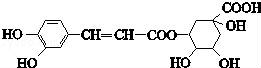
A������ʽΪC16H18O9
B������NaHCO3��Ӧ
C���ܷ���ȡ����Ӧ��������Ӧ����ȥ��Ӧ
D��1 mol��ԭ�������6 mol Br2��Ӧ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com