
ij��ѧ��ȤС����ʵ������ȡƯ�ۣ���̽��������ʯ���鷴Ӧ�������Ͳ��
��֪���ٶ���������Ũ���ᷴӦ���Ʊ�������ͬʱ����MnCl2��
�������ͼ�ķ�Ӧ�Ĺ����зų��������¶Ƚϸ�ʱ�������ͼ�ܷ������·�Ӧ��
6Cl2 + 6Ca(OH)2  5CaCl2 + Ca(ClO3)2 + 6H2O
5CaCl2 + Ca(ClO3)2 + 6H2O
����ȤС�����������ʵ��װ�ã�����ʵ�顣
 |
�� �� �� ��
��ش��������⣺
��1���ټ�װ���з�����Ӧ�Ļ�ѧ����ʽ�� ��
����װ���е��Լ��� �������� ��
�۸���ȤС����300mL 12mol/L������17.4g MnO2�Ʊ��������������������������ʯ���鷴Ӧ���������������Ƶñ�������� L��Ca(ClO)2 g��
 ��2��С���Ա���֣�������Ca(ClO)2����������С��
��2��С���Ա���֣�������Ca(ClO)2����������С��
����ֵ���������ۺ���Ϊ����������δ��ʯ����
��Ӧ���ݳ����Լ��¶������ǿ���ԭ��Ϊ��̽
����Ӧ�����Բ����Ӱ�죬������ȡһ������ʯ
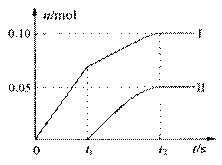
���飬���������ٵ�ͨ�������������ó���ClO����
ClO3�� �������ӵ����ʵ�����n���뷴Ӧʱ�䣨t��
�Ĺ�ϵ���ߣ����Ա�ʾΪ��ͼ��������������ˮ
�ķ�Ӧ����
��ͼ�����ߢ��ʾ ���ӵ����ʵ����淴Ӧʱ��仯�Ĺ�ϵ��
����ȡʯ�����к���Ca(OH)2�����ʵ���Ϊ mol��
 ����ȡһ����ڵ����ʵ���Ca(OH)2��ʯ���飬�Խϴ������ͨ��������������Ӧ���ò�����Cl�������ʵ���Ϊ0.35mol��������� = ��
����ȡһ����ڵ����ʵ���Ca(OH)2��ʯ���飬�Խϴ������ͨ��������������Ӧ���ò�����Cl�������ʵ���Ϊ0.35mol��������� = ��
���𰸡�
��1���� 4HCl (Ũ) + MnO 2  MnCl2 + C12�� + 2H2O��
MnCl2 + C12�� + 2H2O��
�� ����ʳ��ˮ�� ��ȥ�����л��е��Ȼ��⣻
�� 4.48�� 14.3��
��2���� ClO3�� ��0.7 �� 2��1
��������
��1����4HCl (Ũ) + MnO 2  MnCl2 + C12�� + 2H2O
MnCl2 + C12�� + 2H2O
�����е��Լ��DZ���ʳ��ˮ��Ŀ���dz�ȥ�����л��е��Ȼ���
�������������MnO2���㣬�ɵ�0.2mol��������4.48L�����ݷ���ʽ2mol������143g Ca(ClO)2��0.2mol������14.3g Ca(ClO)2.
��2���� ��t1ʱ�ų������ӣ����ʾ���ɵ���ClO3��
�ڢ��������Ca(ClO)2.���������Ca(ClO3)2�����ݵ�ʧ�����غ��n��CaCl2��=0.35mol.���ݸ�Ԫ���غ��Ca(OH)2�����ʵ���Ϊ0.7mol��
��Cl�������ʵ���Ϊ0.35mol�����ݵ�ʧ�����غ����������������ı�ֵΪ2��1��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����C��H��O�Ļ������C��H��O��������Ϊ12��1��16��������������������ܶ�Ϊ58������ˮ��Һ��ʹ���ȱ�죬0.58g������������50mL0.2mol/L������������Һ��ȫ��Ӧ������ʹ��ˮ��ɫ���ƶ��������ʵĽṹ��ʽ��Ҫ��д���ƶϹ��̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���� X��
X��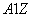 X���������ӣ�����������ȷ����
X���������ӣ�����������ȷ����
A�����������
B��XԪ�ص�������ΪA
C�� X��һ������ϡ������Ԫ��ԭ�ӵĺ�������Ų�
X��һ������ϡ������Ԫ��ԭ�ӵĺ�������Ų�
D�����ǵĻ�ѧ���ʼ�����ȫ��ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
V LŨ��Ϊ1 mol��L��1�����ᣬ��ʹ��Ũ������1������ȡ�Ĵ�ʩ��������
A��ͨ���״���µ�HCl����22.4V L
B������Һ����Ũ����0.5V L
C������10 mol��L��1������0.2V L����ϡ����1.5V L
D������V L 3 mol��L��1�������Ͼ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NA��ʾ����٤��������ֵ������˵������ȷ�� �� ��
A�������£�42 g C2H4��C4H8�Ļ�����к��е�̼ԭ����Ϊ3 NA
B��58.5 g�Ȼ��ƹ����к���NA���Ȼ��Ʒ��ӣ�
C������£�11.2 L���������ķ�����Ϊ0.5 NA��
D��1 mol FeCl3��ȫˮ��ת��Ϊ�����������������NA��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������Ȼ�ѧ����ʽ�ó��Ľ�����ȷ���� �� �� (����)��
A����֪2H2(g)��O2(g)===2H2O(g)����H����483.6 kJ·mol��1����������ȼ����Ϊ241.8 kJ·mol��1
B�� ��֪NaOH(aq)��HCl(aq)===NaCl(aq)��H2O(l)����H����57.3 kJ·mol��1����40.0 g NaOH��ϡ��Һ��ϡ������ȫ�кͣ��ų�57.3 kJ������
C����֪2C(s)��2O2(g)===2CO2(g)����H��a��2C(s)��O2(g)===2CO(g)����H��b����a>b
D����֪C(ʯī��s)===C(���ʯ��s)����H>0����ʯī�Ƚ��ʯ�ȶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
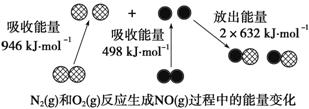
��ѧ��Ӧ�е������仯���ɻ�ѧ��Ӧ�оɻ�ѧ������ʱ���յ�
�������»�ѧ���γ�ʱ�ų���������ͬ����ġ�����ͼΪN2(g)
��O2(g)��Ӧ����NO(g)�����е������仯��
����˵����ȷ���ǣ� ��
A��1 mol N2(g)��1 mol O2(g)��Ӧ�ų�������Ϊ180 kJ
B��1 mol N2(g)��1 mol O2(g)�����������2 mol NO(g)���������
C��ͨ������£�N2(g)��O2(g)�����ֱ������NO
D��NO��һ���������������NaOH��Һ��Ӧ�����κ�ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��̬��A��H2������ܶ�Ϊ14�������������������һ�����ҵ�ʯ�ͻ�����չˮƽ���Ըû�����Ϊԭ�Ϻϳɻ�����G��E��I���������£�
��֪�������廯����FΪC��H��O���������Է�������Ϊ166�����ϵ�һ�ȴ�����һ�֣�1 mol F������NaHCO3��Һ��Ӧ������2 mol CO2��F������B��Ӧ����G��
��HΪ��Ԫ�����������ܶ�����ɱ�״��Ϊ2.77 g/L��H������D��Ӧ����I��

(1)A�й����ŵ�����Ϊ ��E�Ľṹ��ʽ ��
(2)G�ķ���ʽΪ ����Ӧ�ݵķ�Ӧ����Ϊ ��
(3)д�����л�ѧ����ʽ��
�� ��
�� ��
(4)F��H�����ɸ߷��ӻ�����J��д������J�Ļ�ѧ��Ӧ����ʽ��
��
(5)I�ж���ͬ���칹�壬����һ��ͬ���칹��������������
�ٷ����к�����Ԫ���ṹ����1 mol���л���������NaHCO3��Һ��Ӧ��������1 mol CO2����1 mol���л���������Na��Ӧ��������1.5 mol H2���ܻ��ϵ�һ�ȴ���ֻ�����֡������������������л�������п��ܵĽṹ��ʽΪ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com