
�й�������ŵ,��2020��,��λGDP������̼�ŷű�2005���½�40%��50%��
��1����Ч��̼���ֶ�֮һ�ǽ���,�������ⷽ������ܵ�������������
A.���ˮ����:2H2O 2H2��+O2��
2H2��+O2��
B.����ʹˮ�ֽ�����:2H2O 2H2��+O2��
2H2��+O2��
C.̫������ֽ�ˮ����:2H2O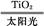 2H2��+O2��
2H2��+O2��
D.��Ȼ������:CH4+H2O CO+3H2
CO+3H2
��2��CO2��ת�����л���ʵ��̼ѭ���������Ϊ1 L���ܱ�������,����1 mol CO2��3 mol H2,һ�������·�Ӧ:CO2��g��+3H2��g�� CH3OH��g��+H2O��g������H="-49.0" kJ/mol,���CO2��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ��
CH3OH��g��+H2O��g������H="-49.0" kJ/mol,���CO2��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ��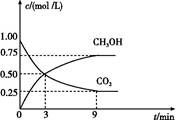
�ٴ�3 min��9 min,v��H2��=��������mol/��L��min����
����˵��������Ӧ�ﵽƽ��״̬�����������������ţ���
A.��Ӧ��CO2��CH3OH�����ʵ���Ũ��֮��Ϊ1��1����ͼ�н���㣩
B.���������ܶȲ���ʱ��ı仯���仯
C.��λʱ��������3 mol H2,ͬʱ����1 mol H2O
D.CO2����������ڻ�������б��ֲ���
��3����ҵ��,CH3OHҲ����CO��H2�ϳɡ��ο��ϳɷ�ӦCO��g��+2H2��g�� CH3OH��g����ƽ�ⳣ��:
CH3OH��g����ƽ�ⳣ��:
| �¶�/�� | 0 | 100 | 200 | 300 | 400 |
| ƽ�ⳣ�� | 667 | 13 | 1.9��1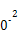 | 2.4��1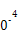 | 1��1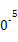 |
 �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д� Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д� �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ��С�ձ������Լ20 g����ĥ�ɷ�ĩ��������������[Ba(OH)2��8H2O]����С�ձ����������ѵ���3��4��ˮ�IJ���Ƭ�ϣ�Ȼ�����ձ��ڼ���Լ10 g�Ȼ�茶��壬�������ò�����Ѹ�ٽ��衣�Իش��������⣺
(1)д����Ӧ�Ļ�ѧ����ʽ�� _____________________________��
(2)ʵ����Ҫ�����ò�����Ѹ�ٽ����ԭ����_____________________________��
(3)���ʵ����û�п���������������ܵ�ԭ����(�����������������ԭ��)__________________________ _____________��
(4)���û�п�����������������ǻ����Բ�ȡ��Щ��ʽ��˵���÷�Ӧ���ȣ�
_______________________________________(������ַ���)��
(5)�����������˵���÷�Ӧ��һ��________(��ų��������ա�)�����ķ�Ӧ�����Ͽ��ɻ�ѧ��________(����ա��ų���)������________(�>����<��)�γ��»�ѧ��________(����ա��ų���)��������
(6)�÷�Ӧ�ڳ����¾Ϳɽ��У�˵��_________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
CH4��H2��C�������ʵ���Դ���ʣ�����ȼ�յ��Ȼ�ѧ����ʽΪ��
��CH4(g)��2O2(g)=CO2(g)��2H2O(l)����H����890.3 kJ��mol��1��
��2H2(g)��O2(g)=2H2O(l)����H����571.6 kJ��mol��1��
��C(s)��O2(g)=CO2(g)����H����393.5 kJ��mol��1��
(1)����д���һ�ּ���ϸ������������øʹ������O2���ò���������������ϸ��ʹ1 mol��������CO2������Һ̬ˮ���ų�������________(���������������)890.3 kJ��
(2)������CO2�����ںϳɺϳ���(��Ҫ�ɷ���һ����̼������)��CH4��CO2=2CO��2H2��1 g CH4��ȫ��Ӧ���ͷ�15.46 kJ����������
����ͼ�ܱ�ʾ�÷�Ӧ�����������仯����________(����ĸ)��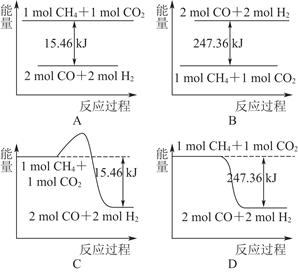
���������ʵ�����Ϊ1 mol��CH4��CO2����ij�����ܱ������У���ϵ�ų�����������ʱ��ı仯��ͼ��ʾ����CH4��ת����Ϊ________��
(3)C(s)��H2(g)����Ӧ������C(s)��2H2(g)=CH4(g)�ķ�Ӧ����ֱ�Ӳ�������ͨ��������Ӧ�������C(s)��2H2(g)=CH4(g)�ķ�Ӧ�Ȧ�H��________��
(4)Ŀǰ���������������ʵ��о���ȼ���о����ص㣬���й��������������ʵ��о������п��е���________(����ĸ)��
| A��Ѱ�����ʴ�����ʹCO2��H2O��Ӧ����CH4��O2�����ų����� |
| B��Ѱ�����ʴ������ڳ��³�ѹ��ʹCO2�ֽ�����̼��O2 |
| C��Ѱ�����ʴ���������̫����ʹ�����е�CO2�뺣���ɵ�CH4�ϳɺϳ���(CO��H2) |
| D������̬̼�ϳ�ΪC60����C60��Ϊȼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ԫ�ص��⻯����������ڹ�ҵ�������������ж��й㷺Ӧ�ã��ش��������⣺
��1����Ԫ��ԭ�ӵ�L�������Ϊ________��
��2���¿���Ϊ�����������ȼ�ϣ���������N2O4��Ӧ����N2��ˮ������
��֪����N2��g����2O2��g��=N2O4��l����
��H1����19.5 kJ��mol��1
��N2H4��l����O2��g��=N2��g����2H2O��g����
��H2����534.2 kJ��mol��1
д���º�N2O4��Ӧ���Ȼ�ѧ����ʽ________________________��
��3����֪H2O��l��=H2O��g������H3����44 kJ��mol��1�����ʾ��ȼ���ȵ��Ȼ�ѧ����ʽΪ________________________��
��4���¡�����ȼ�ϵ����һ�ּ��Ե�أ��õ�طŵ�ʱ�������ķ�ӦʽΪ________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����֪�� C(s)+O2(g)=CO2(g) ��H1����393.5 kJ/mol
C(s)+H2O(g)=CO(g)+H2(g) ��H2����131.3 kJ/mol
��ӦCO(g)+H2(g) +O2(g)= H2O(g)+CO2(g)����H= ____ ___kJ/mol��
��2����һ���ݵ��ܱ������У���CO��H2�ϳɼ״���CO(g)+2H2(g) CH3OH(g) ��H
CH3OH(g) ��H
���������β���˵���÷�Ӧ�Ѵﵽƽ��״̬����_______������ţ���
A��ÿ����1 mol CO��ͬʱ����2molH2
B��������������ʵ�������
C������CH3OH������������CO���������
D��CH3OH��CO��H2��Ũ�ȶ����ٷ����仯
��CO��ƽ��ת���ʣ��������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��A��B�����ƽ�ⳣ��K(A)_______K(B)�����������=������,��ͬ������ͼ�жϦ�H _____0��
��ij�¶��£���2.0 mol CO��6.0 molH2����2 L���ܱ������У���ַ�Ӧ�ﵽƽ��ʱ���c(CO)="0.25" mol/L����CO��ת����= �����¶��µ�ƽ�ⳣ��K= ��������λ��Ч���֣���
��3�������¶�650���������ȼ�ϵ�أ���ú̿����CO��H2����������Ӧ�������CO2�Ļ������Ϊ������Ӧ����������缫����һ��������Li2CO3��Na2CO3���۵�����������ʡ������ĵ缫��ӦʽΪ��CO+H2��4e-+2CO32-=3CO2+H2O����õ�ص�������ӦʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)��֪��H2(g)��1/2O2(g)=H2O(l)����H����285.8 kJ��mol��1
H2(g)=H2(l)����H����0.92 kJ��mol��1
O2(g)=O2(l)����H����6.84 kJ��mol��1
H2O(l)=H2O(g)����H����44.0 kJ��mol��1
��д��Һ���Һ����Ӧ������̬ˮ���Ȼ�ѧ����ʽ��__________________________
�������ҺΪKOH��Һ������ȼ�ϵ�أ��为���ĵ缫��ӦʽΪ____________________________________��
(2)��ͼ��ʾ373 Kʱ����ӦA(g)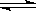 2B(g)��ǰ110 s�ڵķ�Ӧ���̡�
2B(g)��ǰ110 s�ڵķ�Ӧ���̡�
�ٴ˷�Ӧ��ƽ�ⳣ������ʽK��________��
��373 Kʱ��Ӧ���е�70 sʱ���ı������������________����Ӧ���е�90 sʱ���ı������������________��
| A��������� | B������������� | C�������¶� | D������A��Ũ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ĿǰΪֹ���ɻ�ѧ��ת��Ϊ���ܻ������Ȼ������ʹ������Ҫ����Դ��
��1����ѧ��Ӧ�зų������ܣ��ʱ䣬��H���뷴Ӧ����������ڷ�Ӧ�����жϼ����γ��¼����������պͷų������Ĵ�С�йء�
��֪��H2��g����Cl2��g��=2HCl��g�� ��H����185 kJ/mol������1 mol H��H�����յ�����Ϊ436 kJ������1 mol Cl��Cl�����յ�����Ϊ247 kJ�����γ�1 mol H��Cl���ų�������Ϊ ��
��2��ȼ��ȼ�ս��������Ļ�ѧ��ת��Ϊ��������Ҫ�����ܡ���֪��
��CH4��g����2O2��g��=CO2��g����2H2O��l�� ��H����890��3 kJ��mol-1
��C��s,ʯī����O2��g��=CO2��g�� ��H����393��5 kJ��mol��1
��2H2��g����O2��g��=2H2O��l�� ��H����571��6 kJ��mol-1
��״����22��4 L�����ͼ���Ļ�������������������г��ȼ�շ�Ӧ�ų�588��05 kJ��������ԭ��������������������� ���������������Ȼ�ѧ����ʽ������C��s,ʯī����2H2��g��=CH4��g���ķ�Ӧ�Ȧ�HΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������ЧӦ����Դ��ȱ�����⣬��ν��ʹ����е�CO2���������Կ������ã������˸������ձ����ӡ�Ŀǰ��ҵ����һ�ַ�������CO2����ȼ�ϼ״���һ�������·�����Ӧ��CO2(g)+3H2(g) CH3OH(g)+H2O(g)����ͼ��ʾ�÷�Ӧ���й���������(��λΪkJ��mol��1)�ı仯��
CH3OH(g)+H2O(g)����ͼ��ʾ�÷�Ӧ���й���������(��λΪkJ��mol��1)�ı仯��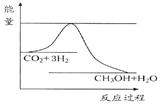
��1�����ڸ÷�Ӧ������˵���У����H 0��(����ڡ�����С�ڡ����ڡ�)�� ���� ����ϸߡ��ϵ͡����¶��������ڸ÷�Ӧ�Է����С�
��2���÷�Ӧƽ�ⳣ��K�ı���ʽΪ ��
��3���¶Ƚ��ͣ�ƽ�ⳣ��K (����������䡱��С��)��
��4����Ϊ�����ݻ���ͬ���ܱ�����,����������г���1 mol CO2(g)��3 molH2(g)���������г���1mol CH3OH(g)��1 mol H2O(g)������ͬ���¶��½��з�Ӧ,�ﵽƽ��ʱ,��������n(CH3OH)���� (����ڡ���С�ڡ����ڡ�)��������n(CH3OH)��
��5����֪��CO(g)+2H2(g) = CH3OH (g) ��H=" -116" kJ?mol-1��CO(g)+1/2O2(g)=CO2(g) ��H="-283" kJ?mol-1��H2 (g)+1/2O2(g)=H2O(g) ��H="-242" kJ?mol-1 ,д��CH3OHȼ������CO2��ˮ�������Ȼ�ѧ����ʽ______________________________________��
��6���Լ״�������Ϊȼ�ϣ�����������ҺΪ�������Һ���ɵ�ء�
�ٸ����ĵ缫��ӦʽΪ ��
������ʯīΪ�缫���������ͭ��Һ��д�������ܷ�Ӧ����ʽ �����Դ�ȼ�ϵ�ص��200 mL 0.8mol/L������ͭ��Һ��������1.6�״�ʱ�������������� gͭ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com