
Ā±ĖŲ»Æѧ·įø»¶ą²Ź£¬ÄÜŠĪ³ÉĀ±»ÆĪļ”¢Ā±ĖŲ»„»ÆĪļ”¢¶ąĀ±»ÆĪļµČ¶ąÖÖĄąŠĶµÄ»ÆŗĻĪļ”£
£Ø1£©»łĢ¬äåŌ×ӵļŪµē×ÓÅŲ¼Ź½ĪŖ ”£
£Ø2£©Ā±ĖŲ»„»ÆĪļČēIBr”¢IClµČÓėĀ±ĖŲµ„ÖŹ½į¹¹ĻąĖĘ”¢ŠŌÖŹĻą½ü”£ŌņCl2”¢IBr”¢IClµÄ·ŠµćÓÉøßµ½µĶµÄĖ³ŠņĪŖ ”£
£Ø3£©ĘųĢ¬·ś»ÆĒāÖŠ“ęŌŚ¶ž¾Ū·Ö×Ó(HF)2£¬ÕāŹĒÓÉÓŚ ”£
£Ø4£©»„ĪŖµČµē×ÓĢåµÄĪ¢Į£Ļą»„Ö®¼ä½į¹¹ĻąĖĘ”£I3+ŹōÓŚ¶ąĀ±ĖŲŃōĄė×Ó£¬øł¾ŻVSEPRÄ£ŠĶĶĘ²āI3+µÄæռ乹ŠĶĪŖ £¬ÖŠŠÄŌ×ÓŌÓ»ÆĄąŠĶĪŖ ”£
£Ø5£©¢ŁHClO4”¢¢ŚHIO4”¢¢ŪH5IO6£ŪæÉŠ“³É(HO)5IO£ŻµÄĖįŠŌÓÉĒæµ½ČõµÄĖ³ŠņĪŖ £ØĢīŠņŗÅ£©”£
£Ø6£©Ā±»ÆĪļRbICl2ŌŚ¼ÓČČŹ±»į·Ö½āĪŖ¾§øńÄÜĻą¶Ō½Ļ“óµÄĀ±»ÆĪļAŗĶĀ±ĖŲ»„»ÆĪļ»ņĀ±ĖŲµ„ÖŹ£¬AµÄ»ÆѧŹ½ ”£
£Ø7£©ČēĶ¼ĖłŹ¾ĪŖĀ±»ÆĪļ±ł¾§ŹÆ£Ø»ÆѧŹ½ĪŖNa3AlF6£©µÄ¾§°ū”£Ķ¼ÖŠ”ńĪ»ÓŚ“óĮ¢·½Ģ嶄µćŗĶĆęŠÄ£¬”šĪ»ÓŚ“óĮ¢·½ĢåµÄ12ĢõĄāµÄÖŠµćŗĶ8øöŠ”Į¢·½ĢåµÄĢåŠÄ£¬ØŹĒĶ¼ÖŠ”ń”¢”šÖŠµÄŅ»ÖÖ”£Ķ¼ÖŠ”ń”¢”š·Ö±šÖø“śÄÄÖÖĮ£×Ó ”¢ £»“óĮ¢·½ĢåµÄĢåŠÄ“¦ØĖł“ś±ķµÄŹĒ ”£

£Ø15·Ö£©£Ø1£©4s24p5£Ø2·Ö£© £Ø2£©IBr£¾ICl£¾Cl2£Ø2·Ö£© £Ø3£©HF·Ö×Ó¼äŠĪ³ÉĒā¼ü £Ø2·Ö£©
£Ø4£©VŠĪ£Ø»ņ½ĒŠĪ£©£»sp3£Øø÷1·Ö£¬¹²2·Ö£© £Ø5£©¢Ł£¾¢Ś£¾¢Ū£Ø2·Ö£© £Ø6£©RbCl£Ø2·Ö£©
£Ø7£©”ń£ŗAlF6£ £» ”š£ŗNa£« £» AlF6££Øø÷1·Ö£¬¹²3·Ö£©
”¾½āĪö”æ
ŹŌĢā·ÖĪö£ŗ£Ø1£©äåŌŖĖŲµÄŌ×ÓŠņŹżŹĒ35£¬ĖłŅŌøł¾Ż¹¹ŌģŌĄķŗĶÄÜĮæ×īµĶŌĄķæÉÖŖ£¬»łĢ¬äåŌ×ӵļŪµē×ÓÅŲ¼Ź½ĪŖ4s24p5”£
£Ø2£©Ā±ĖŲµ„ÖŹŠĪ³ÉµÄ¾§Ģå¾łŹĒ·Ö×Ó¾§Ģ壬·Ö×Ó¼ä×÷ÓĆĮ¦Ō½“󣬷ŠµćŌ½øß”£ŌņCl2”¢IBr”¢ICl·ŠµćÓÉó{µ½µĶµÄĖ³ŠņĪŖIBr£¾ICl£¾Cl2”£
£Ø3£©ÓÉÓŚFµÄµēøŗŠŌ×ī“ó£¬Ņņ“ĖHF·Ö×ÓÖŠ“ęŌŚĒā¼ü£¬“Ó¶ųŠĪ³É¶ž¾Ū·Ö×Ó”£
£Ø4£©ŅņĪŖI3+æÉ擳ÉII2+£¬ĖłŅŌøł¾Ż¼Ū²ćµē×Ó¶Ō»„³āĄķĀŪæÉÖŖ£¬I3+ÖŠÖŠŠÄŌ×Óŗ¬ÓŠµÄ¹Ā¶Ōµē×Ó¶ŌŹż£½£Ø7£1£2”Į1£©”Ā2£½2£¬ĖłŅŌĘäæռ乹ŠĶĪŖVŠĪ£¬ĘäÖŠIŌ×ÓµÄŌӻƹģµĄĄąŠĶŹĒsp3ŌӻƔ£
£Ø5£©ÓÉÓŚ·Ē½šŹōŠŌCl£¾Br£¾I£¬·Ē½šŹōŠŌŌ½Ē棬Ōņ¶ŌÓ¦µÄ×īøß¼ŪŃõ»ÆĪļµÄĖįŠŌŌ½Ē棬Ōņ¢ŁµÄĖįŠŌ×īĒ攣ŌŚ¢Ś¢ŪÖŠ¶¼ŹĒµāŌŖĖŲµÄŗ¬ŃõĖį£¬·ĒōĒ»łŃõøöŹżŌ½¶ą£¬ĖįŠŌŌ½Ē棬ŌņĖįŠŌĪŖ¢Ś£¾¢Ū£¬¹ŹČżÖÖĪļÖŹµÄĖįŠŌÓÉĒæµ½ČõµÄĖ³ŠņĪŖ¢Ł¢Ś¢Ū”£
£Ø6£©RbICl2¼ÓČČŹ±»į·Ö½āĪŖ¾§øńÄÜĻą¶Ō½Ļ“óµÄĀ±»ÆĪļAŗĶĀ±ĖŲ»„»ÆĪļ»ņĀ±ĖŲµ„ÖŹ£¬ĀČĄė×ӵİė¾¶Š”ÓŚµāĄė×ӵİė¾¶£¬ŌņRbClµÄĄė×Ó¼ü³¤Š”ÓŚRbIµÄĄė×Ó¼ü³¤£¬ŌņRbClµÄ¾§øńÄܽĻ“ó£¬ĖłŅŌŌņAĪŖRbCl”£
£Ø7£©øł¾Ż¾§°ū½į¹¹²¢ŅĄ¾Ż¾łĢÆ·ØæÉÖŖ£¬”ń±ķŹ¾µÄĪ¢Į£øöŹż£½8”Į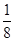 £«6”Į
£«6”Į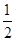 £½4øö£¬”š±ķŹ¾µÄĪ¢Į£øöŹż£½12”Į
£½4øö£¬”š±ķŹ¾µÄĪ¢Į£øöŹż£½12”Į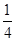 £«8£½11”£øł¾Ż»ÆѧŹ½æÉÖŖ£¬Na+ŗĶAlF6£µÄøöŹż±ČŹĒ3:1£¬Ōņ°×É«ĒņŗĶŗŚÉ«ĒņÖ®±Č½Ó½ü3:1£¬ŅŖĀś×ć3:1£¬ŌņØĖł“ś±ķµÄÓ¦øĆŹĒAlF6£”£
£«8£½11”£øł¾Ż»ÆѧŹ½æÉÖŖ£¬Na+ŗĶAlF6£µÄøöŹż±ČŹĒ3:1£¬Ōņ°×É«ĒņŗĶŗŚÉ«ĒņÖ®±Č½Ó½ü3:1£¬ŅŖĀś×ć3:1£¬ŌņØĖł“ś±ķµÄÓ¦øĆŹĒAlF6£”£
æ¼µć£ŗæ¼²éŗĖĶāµē×ÓÅŲ¼”¢Ēā¼ü”¢æռ乹ŠĶ”¢ŌӻƹģµĄĄąŠĶ”¢ŗ¬ŃõĖįĖįŠŌ”¢·Šµć±Č½ĻŅŌ¼°¾§Ģå½į¹¹µÄÓŠ¹Ų¼ĘĖćµČ



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
 £¬YŌŖĖŲŌ×ӵļŪµē×ÓÅŲ¼Ź½ĪŖ3s2£¬øĆ¾§ĢåµÄŅ»øö¾§°ūČēĶ¼±ūĖłŹ¾£¬ŌņøĆ¾§ĢåµÄ»ÆѧŹ½ĪŖ
£¬YŌŖĖŲŌ×ӵļŪµē×ÓÅŲ¼Ź½ĪŖ3s2£¬øĆ¾§ĢåµÄŅ»øö¾§°ūČēĶ¼±ūĖłŹ¾£¬ŌņøĆ¾§ĢåµÄ»ÆѧŹ½ĪŖ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā


²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ


²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com