
����ѧ����ѡ��3���ʽṹ�����ʡ���15�֣�
A��B��C��D��E��FΪ���ڱ���ǰ�����ڵ�����Ԫ�أ����ǵ�ԭ���������������Ҿ���ͬ�塣A��B��C����Ԫ�صĻ�̬ԭ�Ӿ�����ͬ���ܲ���ܼ����ҵ�һ������I1(A)��I1(C)��I1(B)��BC2+��AC2��Ϊ�ȵ����壻D��EΪͬ��������Ԫ�أ�FԪ��λ�����ڱ���1��18���еĵ�11�С���ش��������⣨����ʱ������Ӧ��Ԫ�ط��ű�ʾ��ӦԪ�أ���
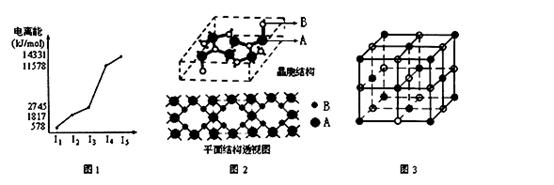
��AԪ�صļ۲�����Ų�ͼΪ ��BC2+�ĵ���ʽΪ �� ��̬��ԭ�ӵĺ�������Ų���ʽ ��
��EԪ�صĵ����������ͼ1��ʾ��EԪ�������ڱ���λ�� �� ��Ԫ�ص����γɵ�
���徧���������� �ѻ���
��A��B��Ԫ�����γɵĻ������������һָ�ij�Ӳ�²��ϣ��侧���ṹ��ͼ2��ʾ���ɴ˿�֪��������
�ľ�������Ϊ ����Ӳ�ȳ������ʯ��ԭ���� ��������ľ�����Bԭ�ӵ��ӻ���ʽΪ ��
��D��C��Ԫ�ؿ��γɵĻ������ҡ�
����֤ʵ�������ҵľ���ṹ��ͼ3��ʾ��������D���ӵ���λ��Ϊ ��
����D���Ӱ뾶�ֱ�Ϊa��C���Ӱ뾶�ֱ�Ϊb�����侧��Ŀռ�������Ϊ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ҽѧ�ϳ������Ը��������Һ�Ͳ�����Һ�ķ�Ӧ���ڲⶨѪ�Ƶĺ�
�����ش��������⣺
 ___H�� + ___MnO4�� + ___H2C2O4 �� ___CO2��+___Mn2�� +____ �� ��
___H�� + ___MnO4�� + ___H2C2O4 �� ___CO2��+___Mn2�� +____ �� ��
 (1).��ƽ�������ӷ���ʽ������ �� �� ���������������
(1).��ƽ�������ӷ���ʽ������ �� �� ���������������
 (2).�÷�Ӧ�еĻ�ԭ����
(2).�÷�Ӧ�еĻ�ԭ����
 (3).��Ӧת����0.4mol���ӣ�������KMnO4�����ʵ���Ϊ mol��
(3).��Ӧת����0.4mol���ӣ�������KMnO4�����ʵ���Ϊ mol��
 (4).�ⶨѪ�Ƶĺ����ķ����ǣ�ȡ2mLѪҺ������ˮϡ�ͺ������м�������(NH4)2C2O4��Һ����Ӧ����CaC2O4��������������ϡ�����ܽ�õ�H2C2O4������KMnO4��Һ�ζ���
(4).�ⶨѪ�Ƶĺ����ķ����ǣ�ȡ2mLѪҺ������ˮϡ�ͺ������м�������(NH4)2C2O4��Һ����Ӧ����CaC2O4��������������ϡ�����ܽ�õ�H2C2O4������KMnO4��Һ�ζ���
 ��ϡ�����ܽ�CaC2O4�����Ļ�ѧ����ʽ��
��ϡ�����ܽ�CaC2O4�����Ļ�ѧ����ʽ��
 ���ܽ����ʱ ���ܻ��ܣ���Ũ���ᣬԭ����
���ܽ����ʱ ���ܻ��ܣ���Ũ���ᣬԭ����
 ����������1.0��10��4mol/L��KMnO4��Һ20.00mL����100mL��ѪҺ�к��� g
����������1.0��10��4mol/L��KMnO4��Һ20.00mL����100mL��ѪҺ�к��� g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ж�������ȷ�Ļ����̡�������Ļ�������
(1)���ʹ��ǽ�̫����ת��Ϊ���ܵij��ò��� (����)
(2012���¿α�ȫ������8B)
(2)�ϳ���ά���ά�����������ǽ������� (����)
(2012���¿α�ȫ������8D)
(3)��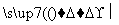 ��
��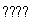 ��������Ϊ��̿��������O2 (����)
��������Ϊ��̿��������O2 (����)
(2013�����գ�6A)
(4)SiO2����HF��Ӧ����������ܱ����ڲ���ƿ�� (����)
(2013���㶫���ۣ�10D)
(5)�������ý�̿��ԭSiO2��ȡ�ֹ� (����)
(2013���㶫���ۣ�11C)
(6)SiO2�е����ԣ�����SiO2�������Ʊ����ά (����)
(2012���㶫���ۣ�12B)
(7)��������ҺӦ�����ڴ����������Լ�ƿ�� (����)
(2012�����ϣ�4B)
(8)ˮ���������������ϼ��ͷ���� (����)
(2010�����գ�4B)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������(Si3N4)��һ�������ĸ��½ṹ�մɣ��ڹ�ҵ�����ͿƼ�����������Ҫ��;��
��.��ҵ���ж��ַ������Ʊ������裬�����Ǽ��ֳ����ķ�����
����һ��ֱ�ӵ���������1 300��1 400 ��ʱ���ߴ���״���봿�������ϣ��䷴Ӧ����ʽΪ________________________________________________________________________��
����������ѧ������������ڸ����������������Ȼ������塢��������������Ӧ���ɵ������HCl���뷽��һ��ȣ��ô˷��Ƶõĵ����贿�Ƚϸߣ���ԭ����________________________________________________________________________��
��������Si(NH2)4�ȷֽⷨ���������Ȼ����백����Ӧ����Si(NH2)4��һ������________________(�����ʽ)��Ȼ��ʹSi(NH2)4���ȷֽ⣬�ֽ�����һ�ֲ���ķ���ʽΪ____________________��
��.(1)�����迹��ʴ������ǿ�����ױ�����ḯʴ��������������ᷴӦ�����ķ������һ����Σ������д��ڵĻ�ѧ��������______________��
(2)��֪��25 �棬101 kPa�����µ��Ȼ�ѧ����ʽ��
3Si(s)��2N2(g)===Si3N4(s)����H����750.2 kJ��mol��1��
Si(s)��2Cl2(g)===SiCl4(g)����H����609.6 kJ��mol��1��
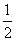 H2(g)��
H2(g)��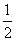 Cl2(g)===HCl(g)����H����92.3 kJ��mol��1��
Cl2(g)===HCl(g)����H����92.3 kJ��mol��1��
��д�����Ȼ��������뵪����������Ӧ���Ȼ�ѧ����ʽ��
________________________________________________________________________��
��.��ҵ����ȡ�ߴ�������Ȼ���������������£�

��֪��X���ߴ��衢ԭ��B����Ҫ�ɷֶ�����Z��Ӧ��Y��X�ڹ��ջ��ȼ�����¿ɷ�Ӧ��Z����ɫ�ʻ�ɫ��
(1)ԭ��B����Ҫ�ɷ���________(д����)��
(2)д����̿��ԭ��B�е���Ҫ�ɷַ�Ӧ�Ļ�ѧ����ʽ��
________________________________________________________________________��
(3)�������������е��A��ˮ��Һʱ�����������ܷ���Cu��________(��ܡ����ܡ�)��д��CuΪ�������A��ˮ��Һ��ʼһ��ʱ�����������ĵ缫����ʽ��
������________������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪25 ��ʱ�й�����ĵ���ƽ�ⳣ����
| ���ữѧʽ | CH3COOH | HCN | H2CO3 |
| ����ƽ�ⳣ��(25 ��) | 1.8��l0��5 | 4.9��l0��10 | K1��4.3��l0��7 K2��5.6��l0��11 |
�ɴ˿ɵó�
A����Ӧ������ҺpH��ϵΪ�� pH(Na2CO3) �� pH(NaCN) �� pH(CH3COONa)
B��CO2ͨ��NaCN��Һ���У�CO2��H2O��2NaCN��Na2CO3��2HCN
C������������μ�ˮ����Һ�ĵ����ԡ�pH����������С
D��NaHCO3��Na2CO3�����Һ�У�һ����c(Na��)��c(H+)��c(OH��)��c(HCO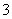 ) ��2c(CO
) ��2c(CO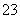 )
)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Mn2����������Һ�еμӹ��������(K2S2O8)��Һ���ᷢ�����·�Ӧ(δ��ƽ)��Mn2����S2O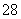 ��H2O�D��MnO
��H2O�D��MnO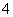 ��SO
��SO ��H��������˵����ȷ����(����)
��H��������˵����ȷ����(����)
A���÷�Ӧ�����������õ���Mn2��
B����Ӧ����Һ��pH����
C����Ӧ����1 mol��ԭ���μӷ�Ӧ����ת�Ƶĵ���Ϊ4 mol
D���÷�Ӧ�����ڼ�����Һ��Mn2���Ĵ��ڣ���������Һ�Ϻ�ɫ��ʧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и���������ָ���������ܴ����������(����)
��Ư�۵�ˮ��Һ�У�Fe2����Cl����Ca2����Na�����ڵμ�ʯ����Һ�ʺ�ɫ����Һ��K����NH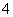 ��Cl����S2�������ܹ������Cu�����·�Ӧ�ų��������Һ��Fe3����Al3����SO
��Cl����S2�������ܹ������Cu�����·�Ӧ�ų��������Һ��Fe3����Al3����SO ��K��
��K��
�ܳ�����pH��2����Һ�У�NH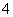 ��Na����Cl����Cu2��
��Na����Cl����Cu2��
����ɫ��Һ�У�K����CH3COO����HCO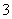 ��MnO
��MnO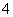
A���ڢ� B���٢�
C���ۢ� D���٢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ�����Ʊ�������װ������ͼ��ͼ���漰���巢�������ӡ�����ռ���β������װ�ã����д������ (����)
A���� B���� C���� D����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ѧ������ѧ�뼼����
��ˮռ�����ܴ�ˮ����97��2�������Ѻ�ˮ�����ͻ�����������������ȿ��Խ����ˮ��Դȱ�������⣬�ֿ��Գ�����ú�����Դ��
(1)�༶��������Ŀǰ����ˮ����������Ҫ�������÷�����һ�������½���ˮ�������������������ȴ���øߴ��ȵ�ˮ���ɴ˿��ж϶༶�������� (������仯�� ��ѧ�仯��)��
(2)���ú�ˮɹ�ε�ԭ���� ������ʳ�ξ�����ĸҺ�к���KCl��MgCl2���������롢�ᴿ������ ��
(3)���ȼҵ�����õ�ⱥ��ʳ��ˮ�Ƶ���Ҫ������Ʒ�����ȼҵ�У���Ĥ�����(��ͼ����ʾ)���������ӽ���Ĥ���(��ͼ����ʾ)����ȡ����
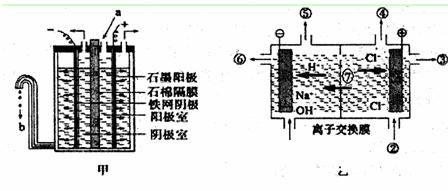
��д�����缫�ķ�Ӧʽ������ ������ ��
��ʯ��Ĥ�������� �����ӽ���Ĥ�����Тޡ��߷ֱ��� �� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com