
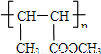 ������л���Ľṹ�������Լ���ĿҪ��ɽ����⣮
������л���Ľṹ�������Լ���ĿҪ��ɽ����⣮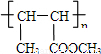 ��
��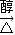 CH2=CHBr+NaBr+H2O��
CH2=CHBr+NaBr+H2O��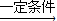
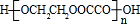 +2��n-1��H2O��
+2��n-1��H2O��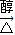 CH2=CHBr+NaBr+H2O��HOCH2CH2OH+HOOC-COOH
CH2=CHBr+NaBr+H2O��HOCH2CH2OH+HOOC-COOH
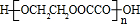 +2��n-1��H2O��
+2��n-1��H2O��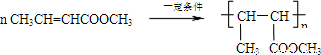 ��
��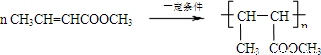 ��
��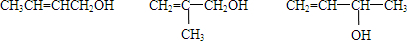 ��
��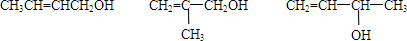 ��
��

 ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�




| �� |
| �� |
| �� |
| �� |




�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



| �� |
| �� |
| �� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| �� |
| �� |
| �� |
| �� |
| һ������ |
 +2��n-1��H2O
+2��n-1��H2O| һ������ |
 +2��n-1��H2O
+2��n-1��H2O



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������д������һ��ѧ�����ڶ����¿���ѧ�Ծ����������� ���ͣ������
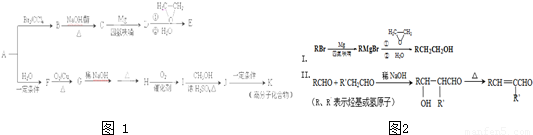
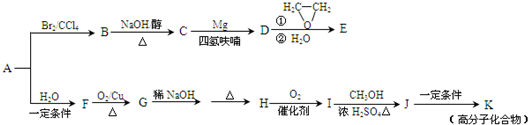
��15�֣���A��һ����Ҫ�Ļ�������ԭ�ϣ��������������Է�������Ϊ28����ͼ����AΪԭ�Ϻϳ�ҩ���м���E����֬K��·�ߡ�
��֪��I. 
II. 
��R��R����ʾ��������ԭ�ӣ�
��1��A�й����ŵĽṹ��ʽ�� ���л���B������
��2��B��C�Ļ�ѧ����ʽΪ ��B���������Ƶ�ˮ��Һ���ȷ�Ӧ���õ����л�������Ҷ��ᷴӦ���ɸ߷��ӻ����д�����ɸ߷��ӻ����ﷴӦ�Ļ�ѧ����ʽ
��3��E�ķ���ʽΪC4H8O�����й���E��˵����ȷ���� ������ĸ��ţ���
a. ��������Ʒ�Ӧ b. ������4��̼ԭ��һ����ƽ��
c. һ�������£�����Ũ�����ᷴӦ d. ��CH2=CHCH2OCH2CH3��Ϊͬϵ��
��4��G��H�漰���ķ�Ӧ������ ��
��5��I�ķ���ʽΪC4H6O2����ṹ��ʽΪ ��
��6��J��K�Ļ�ѧ����ʽΪ ��
��7��д����E������ͬ�����ŵ�����ͬ���칹��Ľṹ��ʽ��
��������˳���칹�������ǡ�OH����˫��̼�ϵĽṹ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ�����и���3��ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ������
��A��һ����Ҫ�Ļ�������ԭ�ϣ��������������Է�������Ϊ28����ͼ����AΪԭ�Ϻϳ�ҩ���м���E����֬K��·�ߡ�

��֪��I. 
II. 
��R��R����ʾ��������ԭ�ӣ�
��1��A�й������� ��E�Ľṹ��ʽ�� ��
��2��B��C�Ļ�ѧ����ʽΪ ��
��3��G��H�漰���ķ�Ӧ������ ��H��I�漰���ķ�Ӧ������ ��
��4��д����E������ͬ�����ŵ�����ͬ���칹��Ľṹ��ʽ��д�����ּ��ɣ���
��������˳���칹�������ǡ�OH����˫��̼�ϵĽṹ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com