
��16�֣���һ��ijͬѧ��һֻ�ձ���װ��һ�����Ĵ����ۣ�����200mL 6mol/L�����ᣬ����ǡ���ܽ⣬��̽����������Ԫ�ؼ�̬��
(1)������裺
����1������ֻ��+2������
����2:___________________________________��
����3:___________________________________��
(2)���ʵ�飺ȡ��Ӧ������Һ�ֱ�װ��ס�����֧�Թܣ��ڼ��еμ�����KMnO4��Һ�������еμ�KSCN��Һ���۲������Ʋ�ʵ����������ۣ�
��������Ϊ____________________�������1��ȷ��
��������Ϊ____________________�������2��ȷ��
��������Ϊ____________________�������3��ȷ��
���������Ȼ����dz�����ˮ����������ҵ���Ʊ���ˮFeCl3������Ϊ��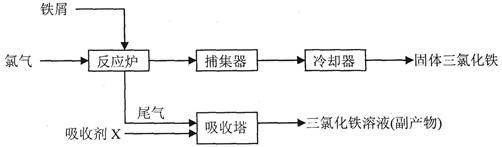
(3)���ռ�XΪFeCl2��Һ������β��Cl2��Ӧ�����ӷ���ʽ__________________��
(4)��ȡ������Ʒm������25mLϡ���ᣬ������ˮ���50mL��Һ�������Թ�����KI��Һ��ַ�Ӧ�� ���õ�����ָʾ������
���õ�����ָʾ������ ��Һ���еζ�
��Һ���еζ� ������Na2S2O3��ҺVmL������Ʒ��FeCl3����������Ϊ____��(���ԭ������Fe��56 Cl��35.5)
������Na2S2O3��ҺVmL������Ʒ��FeCl3����������Ϊ____��(���ԭ������Fe��56 Cl��35.5)
(5)��FeCl3��Һ(32%-35%)��ʴͭ���·ʱ���÷�Һ��FeCl3��FeCl2��CuCl2�����û�ѧ�������շ�Һ�е�ͭ����������Ҫ�㣺___________________________________________________��
(1)����2��������ֻ��+3����Ԫ�أ�1�֣�
����3�������м���+2������+3����Ԫ�ء���1�֣�
(2)�ټ��Թ���Һ��ɫ��ȥ�����Թ�û�����Ա仯����2�֣�
�ڼ��Թ���Һ�����Ա仯�����Թ���Һ���ɫ����2�֣�
�ۼ��Թ���Һ��ɫ��ȥ�����Թ���Һ���ɫ����2�֣�
(3)Cl2��2Fe2��=2Cl����2Fe3����3�֣�
(4)16.25 Vc/m%��3�֣�
(5)�ӹ���Fe�ۣ������ù����ù��������ܽ���������ۣ����ˡ�ϴ�ӡ������2�֣�
����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ijͬѧ��������װ��ʵ��ͭ��Ũ���ᡢϡ���ᷴӦ��������ͼ��ʾ��
ijͬѧ��������װ��ʵ��ͭ��Ũ���ᡢϡ���ᷴӦ��������ͼ��ʾ��| ʵ���� | ˮ��/�� | Һ�������߶� | ||
| 1 | 25 | �����Թܵ�
| ||
| 2 | 50 | �����Թܵ�
| ||
| 3 | 0 | Һ����������ʵ��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�켪��ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
Ϊ��̽��������̬������(SO2��NO2��CO2)�����ʣ�ijͬѧ�����һ��ʵ�飺
ʵ��һ��̽������������ˮ�е��ܽ��ԣ�����֧��ͬ���Թ��ռ����������壬������ʢ��ˮ���ձ��У�һ��ʱ��۲쵽��������ͼA��B��C��ʾ��
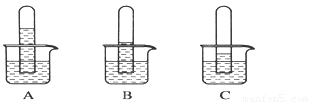
��1������ͬ�����£�����������ˮ���ܽ��������________��д��ѧʽ��д��A�ձ��з�����Ӧ�Ļ�ѧ����ʽ��____ ���������ֻ�ձ��зֱ�μ�����ɫʯ����Һ���ɹ۲쵽��������_____________________ ��
ʵ���������ֻ����ƿ�ռ��������������������壬Ȼ���䵹����ˮ���С��ֱ���ͨ������O2��Cl2����ͼD��E��F��ʾ��һ��ʱ���D��Eװ�õļ���ƿ�г�����Һ��Fװ�õļ���ƿ�л�������ʣ�ࡣ

��2��ʵ�����װ��D�ļ���ƿ���ճ�����Һ(����ƿ��Һ�岻��ɢ)��
��д��װ��D���ܷ�Ӧ�Ļ�ѧ����ʽ��
_______________________________________________��
�ڼ����ʵ�������£�����Ħ�����Ϊa L��mol��1����װ��D�ļ���ƿ��������Һ���ʵ����ʵ���Ũ��Ϊ____________________��
��3�� д��ʵ��Fͨ������������Ӧ�Ļ�ѧ����ʽ��____________________________��
��4����Һ��������ƿ����Eװ�õ�ˮ����μ����ᱵ��Һ�����ܹ۲쵽������Ϊ________�����йص����ӷ���ʽ����ԭ��________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ճ�����������Ľ�����ij��ͬѧ��ѧϰ����֪ʶʱ�����������⣺
����1����Ϊ�γ�Ϊ��ɫ������
����2��CuO�ڸ����¿ɷֽ�ΪCu2O��O2��Fe2O3�ڸ����¿ɷֽ�ΪFeO��O2��
��1����������1��ͬѧ���������ң������ֽ��ͣ�
A.��Ϊ���������к�ɫ��������������Խк�ɫ����
B.��Ϊ���ķ�ĩΪ��ɫ������������Ҳ��Ϊ��ɫ�����Խк�ɫ����
������Ϊ��ȷ��˵����____________��
������һ��ɫ��ĩ����μ��������ۣ�����Fe3O4��ĩ��
____________ ��
������һ��ɫ��ĩ��Ϊ���������������Ļ������֤��������Fe3O4��ֻҪ�����ʵ�鷽������____________ ��
��2����������2��ͬѧ����ʵ��̽�����������������ַ�����
A.�������������������գ�������ǰ����ɫ�Ƿ�仯
B.�������������������գ�������ǰ�������Ƿ�仯
��ʵ����Ӧ��Fe2O3����____________�����������ƣ������ա�
�ڷ���A�У�����������պ���ɫ��____________��Ϊ____________ ��
˵��Fe2O3ȷʵ�����˱仯����˵�����ɵ�һ��ΪFeO��____________����ܡ����ܡ�����������____________��
�۷���B�У����������Ԥ�ڵķ�Ӧ�������������ǰ��������ӦΪ____________�����ǣ�ʵ�����ǹ�������ǰ��������Ϊ30��29����������պ���������____________��
�ܱȽ����ַ���������Ϊ�Ϻõķ�����____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�걱�����и߶����ϣ���ĩ��ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com