
ŌŚŅ»¶ØĪĀ¶ČĻĀĻņĢå»żŗć¶ØĪŖ2LµÄĆܱÕČŻĘ÷Ąļ³äČė2 mol M ŗĶŅ»¶ØĮæµÄN £¬·¢ÉśČēĻĀ·“Ó¦£ŗM (g) £«N(g) E (g) £»µ±·“Ó¦½ųŠŠµ½4minŹ±“ļµ½Ę½ŗā£¬“ĖŹ±²āÖŖMµÄÅضČĪŖ0.2 mol”¤L£1”£ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ
A£®2minŹ±£¬MµÄĪļÖŹµÄĮæÅضČĪŖ0.6mol”¤L£1
B£®4minŹ±£¬ÓĆ£Å±ķŹ¾µÄ·“Ó¦ĖŁĀŹĪŖ0.2mol”¤L£1”¤min£1
C£®4minŗó£¬ĻņČŻĘ÷ÖŠ³äČė²»²ĪÓė·“Ó¦µÄĻ”ÓŠĘųĢ壬MµÄĪļÖŹµÄĮæ²»±ä
D£®4minŹ±£¬MµÄ×Ŗ»ÆĀŹĪŖ80£„

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 £Ø2013?ĮŁćšĻŲÄ£Äā£©ŌŚŅ»¶ØĪĀ¶ČĻĀ£¬Ļņ2LĢå»ż¹Ģ¶ØµÄĆܱÕČŻĘ÷ÖŠ¼ÓČė1molHI£¬2HI?H2£Øg£©+I2£Øg£©”÷H£¾0£¬H2µÄĪļÖŹµÄĮæĖꏱ¼äµÄ±ä»ÆČēĶ¼Ź¾£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©
£Ø2013?ĮŁćšĻŲÄ£Äā£©ŌŚŅ»¶ØĪĀ¶ČĻĀ£¬Ļņ2LĢå»ż¹Ģ¶ØµÄĆܱÕČŻĘ÷ÖŠ¼ÓČė1molHI£¬2HI?H2£Øg£©+I2£Øg£©”÷H£¾0£¬H2µÄĪļÖŹµÄĮæĖꏱ¼äµÄ±ä»ÆČēĶ¼Ź¾£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| ŹµŃé×é | ĪĀ¶Č/”ę | ĘšŹ¼Įæ/mol | Ę½ŗāĮæ/mol | “ļµ½Ę½ŗāĖłŠčŹ±¼ä/min | ||
| CO | H2O | H2 | CO2 | |||
| 1 | 650 | 4 | 2 | 1.6 | 1.6 | 5 |
| 2 | 830 | 1 | 4 | 0.8 | 0.8 | 3 |
| 3 | 830 | a | b | c | d | t |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 H2£Øg£©+CO2£Øg£©µÄĘ½ŗā³£ŹżĖęĪĀ¶ČµÄ±ä»ÆČē±ķ£ŗ
H2£Øg£©+CO2£Øg£©µÄĘ½ŗā³£ŹżĖęĪĀ¶ČµÄ±ä»ÆČē±ķ£ŗ| ĪĀ¶Č/”ę | 400 | 500 | 800 |
| Ę½ŗā³£ŹżKc | 9.94 | 9 | 1 |
| A | B | C | D | E | |
| n£ØCO2£© | 3 | 1 | 0 | 1 | 1 |
| n£ØH2£© | 2 | 1 | 0 | 1 | 2 |
| n£ØCO£© | 1 | 2 | 3 | 0.5 | 3 |
| n£ØH2O£© | 5 | 2 | 3 | 2 | 1 |
 2CO£Øg£©Ę½ŗā³£ŹżK£»
2CO£Øg£©Ę½ŗā³£ŹżK£» CO£Øg£©+H2£Øg£© Ę½ŗā³£ŹżK1£»
CO£Øg£©+H2£Øg£© Ę½ŗā³£ŹżK1£» H2£Øg£©+CO2£Øg£© Ę½ŗā³£ŹżK2£¬
H2£Øg£©+CO2£Øg£© Ę½ŗā³£ŹżK2£¬| K1 |
| K2 |
| K1 |
| K2 |
| 4 |
| 9 |
| 4 |
| 9 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| Ķصē |
| Ņ»¶ØĢõ¼ž |
| c4(NH3)?c3(O2) |
| c2(N2)?c6(H2O) |
| c4(NH3)?c3(O2) |
| c2(N2)?c6(H2O) |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
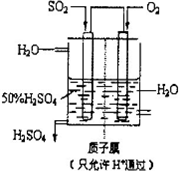 £Ø2010?ĮijĒÄ£Äā£©ĄūÓĆ“ß»ÆŃõ»Æ·“Ó¦½«SO2×Ŗ»ÆĪŖSO3ŹĒ¹¤ŅµÉĻÉś²śĮņĖįµÄ¹Ų¼ü²½Öč£®
£Ø2010?ĮijĒÄ£Äā£©ĄūÓĆ“ß»ÆŃõ»Æ·“Ó¦½«SO2×Ŗ»ÆĪŖSO3ŹĒ¹¤ŅµÉĻÉś²śĮņĖįµÄ¹Ų¼ü²½Öč£®| 1 |
| 2 |
| 10 |
| 3 |
c(C
| ||
c(S
|
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com