
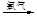
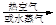 ������
������
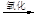
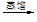 Һ��
Һ�� 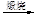
 ʯ����
ʯ����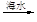
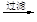
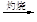 MgO
MgO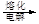 þ
þ
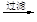 ��Һ
��Һ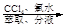 �����л���Һ
�����л���Һ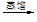 �⾧��
�⾧��
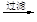
 ����
����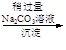
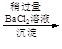
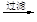 ��Һ
��Һ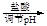
 ʳ�ξ���
ʳ�ξ���
 ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
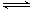 VOA2(�л��㣩+ H2SO4(ˮ��)��������п�ѡ������������ȡ��ԭ����_____________��
VOA2(�л��㣩+ H2SO4(ˮ��)��������п�ѡ������������ȡ��ԭ����_____________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
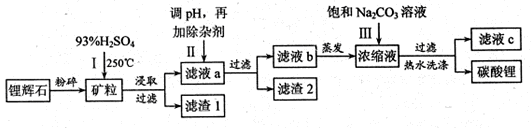
 Li2SO4+Al2O3·4SiO2?H2O
Li2SO4+Al2O3·4SiO2?H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A�������� | B������ | C�������� | D��ʯ���� |
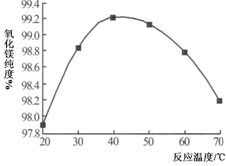
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
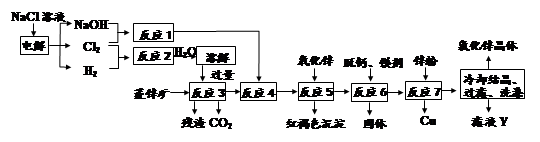
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����¼�������þ��̼�Ļ��������Ƶ���þ |
| B������ұ��������ͨ���û���Ӧ�õ������� |
| C����ˮ����Ĺ����в�����������ԭ��Ӧ |
| D�����õ��ķ������ԴӺ�ˮ�л�õ�ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
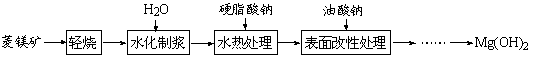
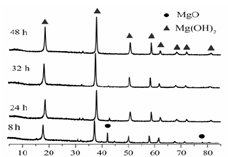
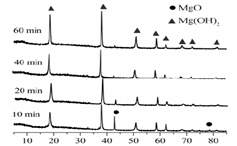
| LiOH | NaOH | KOH | Al(OH)3 | Mg(OH)2 | Ca(OH)2 | Ba(OH)2 |
| 924 | ���ֽ� | ���ֽ� | 140 | 258 | 390 | 700 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���������ܴӺ�ˮ����ȡ��ˮ |
| B���Ӻ�ˮ�п��Եõ��Ȼ�þ���ټ��ȷֽ���ƽ���þ |
| C���������Ӻ�ˮ������Ĺؼ���Ӧ��Cl2+2Br��= 2Cl��+Br2 |
D��ú��������Ҫ��Ӧ��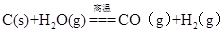 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ˮ | B��п��ϡ���ᷴӦ | C����⺣ˮ | D����ʯ�ͺ���Ȼ��Ϊԭ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com