
£Ø12·Ö£©Ä³ŹµŃ銔×éÓĆ0.50 mol”¤L-1 NaOHČÜŅŗŗĶ0.50 mol”¤L-1ĮņĖįČÜŅŗ½ųŠŠÖŠŗĶČČµÄ²ā¶Ø”£
¢ń£®ÅäÖĘ0.50 mol”¤L-1 NaOHČÜŅŗ
£Ø1£©ČōŹµŃéÖŠ“óŌ¼ŅŖŹ¹ÓĆ245 mL NaOHČÜŅŗ£¬ÖĮÉŁŠčŅŖ³ĘĮæNaOH¹ĢĢå g”£
£Ø2£©“ÓĻĀĶ¼ÖŠŃ”Ōń³ĘĮæNaOH¹ĢĢåĖłŠčŅŖµÄŅĒĘ÷ŹĒ£ØĢī×ÖÄø£©£ŗ ”£
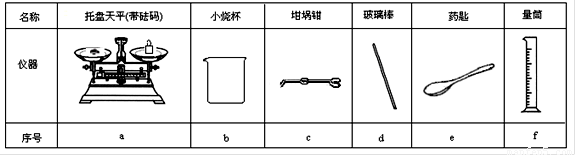
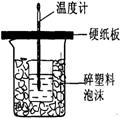
¢ņ£®²ā¶ØĻ”ĮņĖįŗĶĻ”ĒāŃõ»ÆÄĘÖŠŗĶČȵďµŃé×°ÖĆČēÓŅĶ¼ĖłŹ¾”£
¢ņ£®ÖŠŗĶČČµÄ²ā¶Ø£ŗ
£Ø3£©“ÓŹµŃé¹ż³ĢĄ“æ“£¬Ķ¼ÖŠÉŠČ±ÉŁµÄĮ½ÖÖ²£Į§ŅĒĘ÷ŹĒ __”¢________£»
£Ø4£©Č”50 mL NaOHČÜŅŗŗĶ30 mLĮņĖįČÜŅŗ½ųŠŠŹµŃ飬ŹµŃ鏿¾ŻČēĻĀ±ķ”£
¢Ł ĒėĢīŠ“ĻĀ±ķÖŠµÄæÕ°×£ŗ
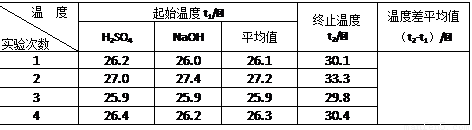
¢Ś ½üĖĘČĻĪŖ0.50 mol/L NaOHČÜŅŗŗĶ0.50 mol/LĮņĖįČÜŅŗµÄĆܶȶ¼ŹĒ1 g/cm3£¬ÖŠŗĶŗóÉś³ÉČÜŅŗµÄ±ČČČČŻc = 4.18 J/(g”¤”ę)”£ŌņÖŠŗĶČČ”÷H = £ØČ”Š”ŹżµćŗóŅ»Ī»£©”£
¢Ū ÉĻŹöŹµŃ鏿ֵ½į¹ūÓė57.3 kJ/molÓŠĘ«²ī£¬²śÉśĘ«²īµÄŌŅņæÉÄÜŹĒ£ØĢī×ÖÄø£© ”£
a£®ŹµŃé×°ÖƱ£ĪĀ”¢øōČČŠ§¹ū²ī
b£®ÅäÖĘ0.50 mol/L NaOHČÜŅŗŹ±ø©ŹÓæĢ¶ČĻ߶ĮŹż
c£®·Ö¶ą“Ī°ŃNaOHČÜŅŗµ¹ČėŹ¢ÓŠĮņĖįµÄŠ”ÉÕ±ÖŠ
d£®ÓĆĪĀ¶Č¼Ę²ā¶ØNaOHČÜŅŗĘšŹ¼ĪĀ¶ČŗóÖ±½Ó²ā¶ØH2SO4ČÜŅŗµÄĪĀ¶Č
e£®ÓĆĮæĶ²ĮæČ”NaOHČÜŅŗµÄĢå»żŹ±ŃöŹÓ¶ĮŹż
£Ø5£©ŹµŃéÖŠøÄÓĆ30 mL 0.50 mol/LµÄĮņĖįøś50mL 0.55 mol/LµÄNaOHČÜŅŗ½ųŠŠ·“Ó¦£¬ÓėÉĻŹöŹµŃéĻą±Č£¬Ėł·Å³öµÄČČĮæ £ØĢī”°ĻąµČ”±»ņ”°²»ĻąµČ”±£©£¬ĖłĒóÖŠŗĶČȵďżÖµ»į __________£ØĢī”°ĻąµČ”±»ņ”°²»ĻąµČ”±£©”£
£Ø¹²12·Ö£©£Ø1£©5.0 £Ø1·Ö£©
£Ø2£©a b e£ØÉŁŃ”»ņ¶ąŃ”¾ł¼ĒĮć·Ö”¢1·Ö£©
£Ø3£©ĮæĶ²ŗĶ»·ŠĪ²£Į§°ō£Øø÷1·Ö£¬¹²2·Ö£©£»£Ø4£©¢Ł 4.0£Ø2·Ö£©¢Ś -53.5 kJ/mol £ØĆ»”°-”±ŗÅ»ņĆ»µ„Ī»¾ł²»øų·Ö”¢2·Ö£©
¢Ū a c d£ØÉŁŃ””¢“ķŃ”¾ł²»øų·Ö”¢2·Ö£©
£Ø5£©²»ĻąµČ”¢ĻąµČ£»£ØĆææÕ1·Ö£¬¹²2·Ö£©
”¾½āĪö”æ£Ø1£©ÓÉÓŚČŻĮæĘæµÄ¹ęøńƻӊ245mlµÄ£¬ĖłŅŌÓ¦øĆÅäÖĘ250ml£¬Ņņ“ĖŠčŅŖĒāŃõ»ÆÄʵÄÖŹĮæŹĒ0.5mol/L”Į0.25L”Į40g/mol£½5.0g”£
£Ø2£©ĒāŃõ»ÆÄĘ¹ĢĢå³ĘĮ棬ŠčŅŖĶŠÅĢĢģĘ½£¬ÉÕ±ŗĶŌæ³×£¬Ņņ“ĖÕżČ·“š°øŃ”abe”£
£Ø3£©æ¼²éÖŠŗĶČČµÄ²ā¶Ø¼°Īó²ī·ÖĪöµČ”£ŌŚŹµŃé¹ż³ĢÖŠ£¬ĪŖŹ¹ČÜŅŗ»ģŗĻ¾łŌČ£¬ŠčŅŖ½Į°č£¬Ņņ“Ė»¹Č±ÉŁµÄŅĒĘ÷ŹĒ»·ŠĪ²£Į§½Į°č°ō”£
£Ø4£©¢Łøł¾Ż±ķÖŠŹż¾ŻæÉÖŖ£¬ĪĀ¶ČµÄ²īÖµŹĒ£Ø”ę£©4.0”¢6.1”¢3.9”¢4.1£¬ĖłŅŌøł¾Ż±ķÖŠŹż¾ŻŹµŃé2µÄĪó²īĢ«“ó£¬ÉįČ„”£Ņņ“ĖĪĀ¶Č²īµÄĘ½¾łÖµŹĒ£Ø4.0+3.9+4.1£©”ę”Ā3£½4.0”ę”£
¢Ś·“Ó¦ÖŠÉś³É0.025molĖ®£¬ĖłŅŌ·“Ó¦ČČ”÷H£½£0.00418”Į4.0”Į80”Ā0.025£½£53.5 kJ/mol”£
¢Ū53.5 kJ/molŠ”ÓŚ57.3 kJ/mol£¬Ń”ĻīaÓŠČČĮæĖšŹ§£¬²ā¶Ø½į¹ūĘ«Š”£»ÅäÖĘ0.50 mol/L NaOHČÜŅŗŹ±ø©ŹÓæĢ¶ČĻ߶ĮŹżŹ±£¬ÅضČĘ«“󣬲ā¶Ø½į¹ūĘ«øߣ»“ĪŹżŌ½¶ą£¬ČČĮæĖšŹ§Ō½¶ą£»ÓĆĪĀ¶Č¼Ę²ā¶ØNaOHČÜŅŗĘšŹ¼ĪĀ¶ČŗóÖ±½Ó²ā¶ØH2SO4ČÜŅŗµÄĪĀ¶Č£¬ŌņĪĀ¶Č²īÖµ¼õŠ”£¬²ā¶Ø½į¹ūĘ«Š”£»ÓĆĮæĶ²ĮæČ”NaOHČÜŅŗµÄĢå»żŹ±ŃöŹÓ¶ĮŹż£¬ŌņĒāŃõ»ÆÄʵÄĢå»żŌö¼Ó£¬²ā¶Ø½į¹ūĘ«øߣ¬ŹµŃé“š°øŃ”acd”£
£Ø5£©øıäĖį»ņ¼īµÄÓĆĮ棬·“Ó¦·Å³öµÄČČĮæøı䣬µ«ÖŠŗĶČČŹĒ²»±äµÄ£¬ŅņĪŖÖŠŗĶČČÓŚĖį»ņ¼īµÄÓĆĮæĪŽ¹ŲĻµ”£



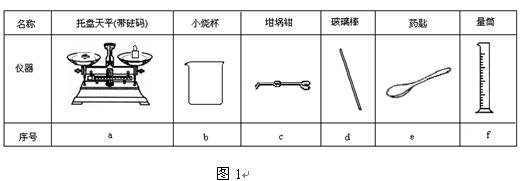
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijŹµŃ銔×éÓĆ0.50 mol/L NaOHČÜŅŗŗĶ0.50 mol/LĮņĖįČÜŅŗ½ųŠŠÖŠŗĶČČµÄ²ā¶Ø”£
¢ń£®ÅäÖĘ0.50 mol/L NaOHČÜŅŗ
£Ø1£©ČōŹµŃéÖŠ“óŌ¼ŅŖŹ¹ÓĆ245 mL NaOHČÜŅŗ£¬ÖĮÉŁŠčŅŖ³ĘĮæNaOH¹ĢĢå g”£
£Ø2£©“ÓĶ¼1ÖŠŃ”Ōń³ĘĮæNaOH¹ĢĢåĖłŠčŅŖµÄŅĒĘ÷ŹĒ£ØĢī×ÖÄø£©£ŗ ”£
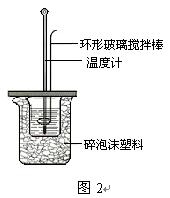
¢ņ£®²ā¶ØĻ”ĮņĖįŗĶĻ”ĒāŃõ»ÆÄĘÖŠŗĶČȵďµŃé×°ÖĆČēĶ¼2ĖłŹ¾”£
£Ø3£©Š“³öøĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½£ØÖŠŗĶČČĪŖ57.3 kJ/mol£©£»
£Ø4£©Č”50 mL NaOHČÜŅŗŗĶ30 mLĮņĖįČÜŅŗ½ųŠŠŹµŃ飬ŹµŃ鏿¾ŻČēĻĀ±ķ”£
¢ŁĒėĢīŠ“ĻĀ±ķÖŠµÄæÕ°×£ŗ
| ĪĀ¶Č ŹµŃé“ĪŹż | ĘšŹ¼ĪĀ¶Čt1/”ę | ÖÕÖ¹ĪĀ¶Č t2/”ę | ĪĀ¶Č²īĘ½¾łÖµ £Øt2-t1£©/”ę | ||
| H2SO4 | NaOH | Ę½¾łÖµ | |||
| 1 | 26.2 | 26.0 | 26.1 | 30.1 | |
| 2 | 27.0 | 27.4 | 27.2 | 33.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | |
¢Ś½üĖĘČĻĪŖ0.50 mol/L NaOHČÜŅŗŗĶ0.50 mol/LĮņĖįČÜŅŗµÄĆܶȶ¼ŹĒ1 g/cm3£¬ÖŠŗĶŗóÉś³ÉČÜŅŗµÄ±ČČČČŻc=4.18 J/(g”¤”ę)”£ŌņÖŠŗĶČČ”÷H= £ØČ”Š”ŹżµćŗóŅ»Ī»£©”£
¢ŪÉĻŹöŹµŃ鏿ֵ½į¹ūÓė57.3 kJ/molÓŠĘ«²ī£¬²śÉśĘ«²īµÄŌŅņæÉÄÜŹĒ£ØĢī×ÖÄø£© ”£
a£®ŹµŃé×°ÖƱ£ĪĀ”¢øōČČŠ§¹ū²ī
b£®ĮæČ”NaOHČÜŅŗµÄĢå»żŹ±ŃöŹÓ¶ĮŹż
c£®·Ö¶ą“Ī°ŃNaOHČÜŅŗµ¹ČėŹ¢ÓŠĮņĖįµÄŠ”ÉÕ±ÖŠ
d£®ÓĆĪĀ¶Č¼Ę²ā¶ØNaOHČÜŅŗĘšŹ¼ĪĀ¶ČŗóÖ±½Ó²ā¶ØH2SO4ČÜŅŗµÄĪĀ¶Č
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğĢģ½ņŹŠĪäĒåĒųŃī“åŅ»ÖŠø߶žµŚŅ»“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗŹµŃéĢā
£Ø12·Ö£©Ä³ŹµŃ銔×éÓĆ0.50 mol”¤L-1 NaOHČÜŅŗŗĶ0.50 mol”¤L-1ĮņĖįČÜŅŗ½ųŠŠÖŠŗĶČČµÄ²ā¶Ø”£
¢ń£®ÅäÖĘ0.50 mol”¤L-1 NaOHČÜŅŗ
£Ø1£©ČōŹµŃéÖŠ“óŌ¼ŅŖŹ¹ÓĆ245 mL NaOHČÜŅŗ£¬ÖĮÉŁŠčŅŖ³ĘĮæNaOH¹ĢĢå g”£
£Ø2£©“ÓĻĀĶ¼ÖŠŃ”Ōń³ĘĮæNaOH¹ĢĢåĖłŠčŅŖµÄŅĒĘ÷ŹĒ£ØĢī×ÖÄø£©£ŗ ”£
¢ņ£®²ā¶ØĻ”ĮņĖįŗĶĻ”ĒāŃõ»ÆÄĘÖŠŗĶČȵďµŃé×°ÖĆČēÓŅĶ¼ĖłŹ¾”£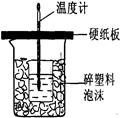
¢ņ£®ÖŠŗĶČČµÄ²ā¶Ø£ŗ
£Ø3£©“ÓŹµŃé¹ż³ĢĄ“æ“£¬Ķ¼ÖŠÉŠČ±ÉŁµÄĮ½ÖÖ²£Į§ŅĒĘ÷ŹĒ __”¢________£»
£Ø4£©Č”50 mL NaOHČÜŅŗŗĶ30 mLĮņĖįČÜŅŗ½ųŠŠŹµŃ飬ŹµŃ鏿¾ŻČēĻĀ±ķ”£
¢ŁĒėĢīŠ“ĻĀ±ķÖŠµÄæÕ°×£ŗ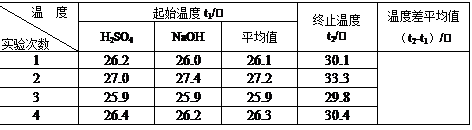
¢Ś½üĖĘČĻĪŖ0.50 mol/L NaOHČÜŅŗŗĶ0.50 mol/LĮņĖįČÜŅŗµÄĆܶȶ¼ŹĒ1 g/cm3£¬ÖŠŗĶŗóÉś³ÉČÜŅŗµÄ±ČČČČŻc =" 4.18" J/(g”¤”ę)”£ŌņÖŠŗĶČČ”÷H= £ØČ”Š”ŹżµćŗóŅ»Ī»£©”£
¢ŪÉĻŹöŹµŃ鏿ֵ½į¹ūÓė57.3 kJ/molÓŠĘ«²ī£¬²śÉśĘ«²īµÄŌŅņæÉÄÜŹĒ£ØĢī×ÖÄø£© ”£
a£®ŹµŃé×°ÖƱ£ĪĀ”¢øōČČŠ§¹ū²ī
b£®ÅäÖĘ0.50 mol/L NaOHČÜŅŗŹ±ø©ŹÓæĢ¶ČĻ߶ĮŹż
c£®·Ö¶ą“Ī°ŃNaOHČÜŅŗµ¹ČėŹ¢ÓŠĮņĖįµÄŠ”ÉÕ±ÖŠ
d£®ÓĆĪĀ¶Č¼Ę²ā¶ØNaOHČÜŅŗĘšŹ¼ĪĀ¶ČŗóÖ±½Ó²ā¶ØH2SO4ČÜŅŗµÄĪĀ¶Č
e£®ÓĆĮæĶ²ĮæČ”NaOHČÜŅŗµÄĢå»żŹ±ŃöŹÓ¶ĮŹż
£Ø5£©ŹµŃéÖŠøÄÓĆ30 mL 0.50 mol/LµÄĮņĖįøś50mL 0.55 mol/LµÄNaOHČÜŅŗ½ųŠŠ·“Ó¦£¬ÓėÉĻŹöŹµŃéĻą±Č£¬Ėł·Å³öµÄČČĮæ £ØĢī”°ĻąµČ”±»ņ”°²»ĻąµČ”±£©£¬ĖłĒóÖŠŗĶČȵďżÖµ»į __________£ØĢī”°ĻąµČ”±»ņ”°²»ĻąµČ”±£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2015½ģŗ£ÄĻŹ”ČżŃĒŹŠø߶žÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌĄķæĘ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŹµŃéĢā
ijŹµŃ銔×éÓĆ0.50 mol/L NaOHČÜŅŗŗĶ0.50 mol/L H2SO4ČÜŅŗ½ųŠŠÖŠŗĶČČµÄ²ā¶Ø”£
¢ń.ÅäÖĘ0.50 mol/L NaOHČÜŅŗ
£Ø1£©ČōŹµŃéÖŠ“óŌ¼ŅŖŹ¹ÓĆ245 mL NaOHČÜŅŗ£¬ŌņÖĮÉŁŠčŅŖ³ĘĮæNaOH¹ĢĢå g”£
£Ø2£©“ÓĻĀĶ¼ÖŠŃ”Ōń³ĘĮæNaOH¹ĢĢåĖłŠčŅŖµÄŅĒĘ÷(ĢīŠņŗÅ) ”£
|
Ćū³Ę |
ĶŠÅĢĢģĘ½(“ųķĄĀė) |
Š”ÉÕ± |
ŪįŪöĒÆ |
²£Į§°ō |
Ņ©³× |
ĮæĶ² |
|
ŅĒĘ÷ |
|
|
|
|
|
|
|
ŠņŗÅ |
a |
b |
c |
d |
e |
f |
¢ņ.²ā¶ØÖŠŗĶČȵďµŃé×°ÖĆČēĶ¼ĖłŹ¾

£Ø1£©Š“³öĻ”ĮņĖįŗĶĻ”ĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦±ķŹ¾ÖŠŗĶČȵÄČČ»Æѧ·½³ĢŹ½(ÖŠŗĶČČŹżÖµĪŖ57.3 kJ”¤mol£1)£ŗ_______________________________________”£
£Ø2£©Č”50 mL NaOHČÜŅŗŗĶ30 mLĮņĖį½ųŠŠŹµŃ飬ŹµŃ鏿¾ŻČēĻĀ±ķ”£
¢ŁĒėĢīŠ“ĻĀ±ķÖŠµÄæÕ°×£ŗ
|
ĪĀ¶Č ŹµŃé“ĪŹż |
³¬Ź¼ĪĀ¶Čt1/”ę |
ÖÕÖ¹ĪĀ¶Čt2/”ę |
Ę½¾łĪĀ¶Č²ī (t2£t1)/”ę |
||
|
H2SO4 |
NaOH |
Ę½¾łÖµ |
|||
|
1 |
26.2 |
26.0 |
26.1 |
30.1 |
|
|
2 |
27.0 |
27.4 |
27.2 |
33.3 |
|
|
3 |
25.9 |
25.9 |
25.9 |
29.8 |
|
|
4 |
26.4 |
26.2 |
26.3 |
30.4 |
¢Śøł¾ŻÉĻŹöŹµŃ鏿¾Ż¼ĘĖć³öµÄÖŠŗĶČČĪŖ53.5 kJ/mol£¬ÕāÓėÖŠŗĶČȵĥķĀŪÖµ57.3 kJ/molÓŠĘ«²ī£¬²śÉśĘ«²īµÄŌŅņæÉÄÜŹĒ(Ģī×ÖÄø)______”£
a£®ŹµŃé×°ÖƱ£ĪĀ”¢øōČČŠ§¹ū²ī
b£®ŌŚĮæČ”NaOHČÜŅŗµÄĢå»żŹ±ŃöŹÓ¶ĮŹż
c£®·Ö¶ą“Ī°ŃNaOHČÜŅŗµ¹ČėŹ¢ÓŠĻ”ĮņĖįµÄŠ”ÉÕ±ÖŠ
d£®ÓĆĪĀ¶Č¼Ę²ā¶ØNaOHČÜŅŗĘšŹ¼ĪĀ¶ČŗóÖ±½Ó²ā¶ØH2SO4ČÜŅŗµÄĪĀ¶Č
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014ѧğ½Ī÷Ź”øßČżÉĻѧʌµŚĖÄ“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
ijŹµŃ銔×éÓĆ0.50 mol”¤L-1 NaOHČÜŅŗŗĶ0.50 mol”¤L-1ĮņĖįČÜŅŗ½ųŠŠÖŠŗĶČČµÄ²ā¶Ø”£
¢ń£®ÅäÖĘ0.50 mol”¤L-1 NaOHČÜŅŗ
£Ø1£©ČōŹµŃéÖŠ“óŌ¼ŅŖŹ¹ÓĆ245 mL NaOHČÜŅŗ£¬ÖĮÉŁŠčŅŖ³ĘĮæNaOH¹ĢĢå”””” ””g”£
£Ø2£©“ÓĻĀĶ¼ÖŠŃ”Ōń³ĘĮæNaOH¹ĢĢåĖłŠčŅŖµÄŅĒĘ÷ŹĒ£ØĢī×ÖÄø£©£ŗ”” ”””””£
|
Ćū³Ę |
ĶŠÅĢĢģĘ½ (“ųķĄĀė) |
Š”ÉÕ± |
ŪįŪöĒÆ |
²£Į§°ō |
Ņ©³× |
ĮæĶ² |
|
ŅĒĘ÷ |
|
|
|
|
|
|
|
ŠņŗÅ |
a |
b |
c |
d |
e |
f |
¢ņ£®²ā¶ØĻ”ĮņĖįŗĶĻ”ĒāŃõ»ÆÄĘÖŠŗĶČȵďµŃé×°ÖĆČēĶ¼ĖłŹ¾”£
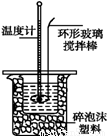
£Ø1£©Š“³öøĆ·“Ó¦ÖŠŗĶČȵÄČČ»Æѧ·½³ĢŹ½£ŗ£ØÖŠŗĶČČĪŖ57.3 kJ”¤mol-1£© ”””” ”””” ”””” ”””” ”£
£Ø2£©Č”50 mL NaOHČÜŅŗŗĶ30 mLĮņĖįČÜŅŗ½ųŠŠŹµŃ飬ŹµŃ鏿¾ŻČēĻĀ±ķ”£
|
ĪĀ¶Č ŹµŃé“ĪŹż”” |
ĘšŹ¼ĪĀ¶Čt1/”ę |
ÖÕÖ¹ĪĀ¶Čt2/”ę |
ĪĀ¶Č²ī Ę½¾łÖµ (t2-t1)/”ę |
||
|
H2SO4 |
NaOH |
Ę½¾łÖµ |
|||
|
1 |
26.2 |
26.0 |
26.1 |
29.6 |
|
|
2 |
27.0 |
27.4 |
27.2 |
31.2 |
|
|
3 |
25.9 |
25.9 |
25.9 |
29.8 |
|
|
4 |
26.4 |
26.2 |
26.3 |
30.4 |
|
¢ŁÉĻ±ķÖŠµÄĪĀ¶Č²īĘ½¾łÖµĪŖ”” ”ę
¢Ś½üĖĘČĻĪŖ0.50 mol”¤L-1 NaOHČÜŅŗŗĶ0.50 mol”¤L-1ĮņĖįČÜŅŗµÄĆܶȶ¼ŹĒ1 g”¤cm-3£¬ÖŠŗĶŗóÉś³ÉČÜŅŗµÄ±ČČČČŻc=4.18 J”¤(g”¤”ę)-1”£ŌņÖŠŗĶČȦ¤H=”””””” £ØČ”Š”ŹżµćŗóŅ»Ī»£©”£
¢ŪÉĻŹöŹµŃ鏿ֵ½į¹ūÓė57.3 kJ”¤mol-1ÓŠĘ«²ī£¬²śÉśĘ«²īµÄŌŅņæÉÄÜŹĒ£ØĢī×ÖÄø£©”””””””””£
a£®ŹµŃé×°ÖƱ£ĪĀ”¢øōČČŠ§¹ū²ī
b£®ĮæČ”NaOHČÜŅŗµÄĢå»żŹ±ŃöŹÓ¶ĮŹż
c£®·Ö¶ą“Ī°ŃNaOHČÜŅŗµ¹ČėŹ¢ÓŠĮņĖįµÄŠ”ÉÕ±ÖŠ
d£®ÓĆĪĀ¶Č¼Ę²ā¶ØNaOHČÜŅŗĘšŹ¼ĪĀ¶ČŗóÖ±½Ó²ā¶ØH2SO4ČÜŅŗµÄĪĀ¶Č
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com