
����Ŀ���������ȣ�ClO2������ɫ������ˮ�����壩�Ǹ�Ч���Ͷ������������ش��������⣺
��1��ʵ������NH4Cl�����ᡢNaClO2���������ƣ�Ϊԭ�ϣ�ͨ�����¹����Ʊ�ClO2��
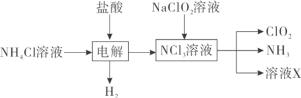
�ٵ��ʱ�����缫��ӦʽΪ__________________________��
�ڳ�ȥClO2�е�NH3��ѡ�õ��Լ���___________�����ţ���
a��ˮ b����ʯ�� c��Ũ���� d������ʳ��ˮ
��2������ͼװ�ÿ��Բⶨ�������ClO2�ĺ�����
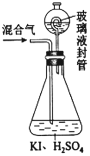
������ƿ�м��������ĵ⻯�أ���50mLˮ�ܽ���ټ���3mLϡ���
���ڲ���Һ��װ���м���ˮ��ʹҺ��û������Һ��ܵĹܿڣ�
��һ�����Ļ������ͨ����ƿ�����գ�
����������Һ��װ���е�ˮ������ƿ�У�
������0.1000mol��L-1��������Ʊ���Һ�ζ���ƿ�е���Һ��I2+2S2O32-��2I��+S4O62-����ָʾ����ʾ�յ�ʱ����ȥ20.00mL�����������Һ���ڴ˹����У�
����ƿ��ClO2��⻯�ط�Ӧ�����ӷ���ʽΪ______________________��
�ڲ���Һ��װ�õ�������______________________��
��V�м���ָʾ�����ζ����յ��������______________________��
�ܲ�û������ClO2������Ϊ______g��
��ijͬѧ��ij���̶ֿ�ģ�������50mL�ζ��ܽ���ʵ�飬���ζ����е�Һ�洦����ͼ��ʾ�Ŀ̶ȴ��������Һ������________������ţ���
a������23.60mL b������27.60mL c����23.60mL d������27.60mL
![]()
���𰸡�NH4+-6e-+3Cl-=NCl3+4H+ c 2ClO2+10I-+8H+=2Cl-+5I2+4H2O ���ղ�����ClO2 ���������һ����������Ʊ���Һ����Һ����ɫ��Ϊ��ɫ���Ұ��������Һ��ɫ���ٸı� 0.027 d
��������
��1�����ʱ���Դ��������������ʧȥ���ӣ�����������Ӧ�����Դ���������������õ����ӷ�����ԭ��Ӧ����������ClO2���Ʊ����̣���֪��������H2��NCl3���ݴ˿ɵó��缫��Ӧ����ʽ��
��2������ʵ����̣���֪ʵ��������漰�ķ�Ӧ����ʽ�У�2ClO2+10I-+8H+=2Cl-+5I2+4H2O��I2+2S2O32-��2I��+S4O62-���ݴ˽��з�����
��1�������ʱ����ʧȥ���ӣ�����������Ӧ��������е��������ɵIJ����֪�����缫��Ӧ����ʽΪ��NH4+-6e-+3Cl-=NCl3+4H+����Ϊ��NH4+-6e-+3Cl-=NCl3+4H+��
������ClO2��NH3�����ʽ��������
a��ClO2��NH3��������ˮ��������ˮ�ͱ���ʳ��ˮ����ȥ������a�����
b����ʯ�Ҳ������հ�����b�����
c��Ũ����������հ������Ҳ�Ӱ��ClO2���ʳ�ȥ��ȥClO2�е�NH3��ѡ�õ��Լ�Ũ���ᡣc����ȷ��
d��ClO2��NH3��������ˮ�������ñ���ʳ��ˮ����ȥ������d�����
����c��
��2�����������Ϣ��֪��ClO2ͨ����ƿ��I-����ΪI2����������ԭΪCl-��ͬʱ����ˮ������ƿ��ClO2��⻯�ط�Ӧ�����ӷ���ʽΪ��2ClO2+10I-+8H+=2Cl-+5I2+4H2O����Ϊ��2ClO2+10I-+8H+=2Cl-+5I2+4H2O��
������ClO2�����壬���ӷ��������У����Բ���Һ��װ�õ����������ղ����ClO2���壬��Ϊ�����ղ�����ClO2��
�����ݵ��������������Һ��Ӧ�Ǽ����˵�����ָʾ����������ζ��յ�ʱ��I2��ȫת��ΪI-����Һ��ɫ��ȥ���ʵζ����յ�������ǣ����������һ����������Ʊ���Һ����Һ����ɫ��Ϊ��ɫ���Ұ��������Һ��ɫ���ٸı䣻��Ϊ�����������һ����������Ʊ���Һ����Һ����ɫ��Ϊ��ɫ���Ұ��������Һ��ɫ���ٸı䣻
�����ݷ�Ӧ����ʽ��2ClO2+10I-+8H+=2Cl-+5I2+4H2O��I2+2S2O32-��2I��+S4O62-���ɵù�ϵʽ��2ClO2��5I2��10S2O32-���ɴ˿�֪���������ClO2������Ϊ![]() ����Ϊ��0.027��
������0.027��
��ͼ��Һ�����Ϊ22.40mL���ζ���������Ϊ50.00mL����Һ�������������Һ�������Ϊ27.60mL����Ϊ�ζ��ܼ��첿������һ����Һ�壬��ζ�����Һ�����Ӧ����27.60mL����ѡd����Ϊ��d��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���⻯���ڹ�ѧ����ʯ��̽�⡢���졢����������������ҪӦ�á�ij�о�С�鿪����Ƶ��Ʊ��ߴ�NaI�ļ�������ͼ��
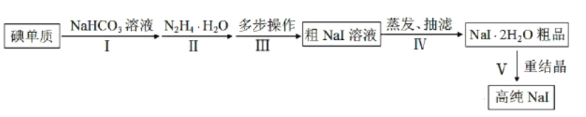
��֪��
��I2(s)��I-(aq)![]() I
I![]() (aq)��
(aq)��
��ˮ����(N2H4H2O)����ǿ��ԭ�ԣ��ɷֱ�ĸ��������I2��ԭΪI-������������Ϊ�����ʡ�
��NaI������ˮ��Ҳ�����ھƾ����ھƾ��е��ܽ�����¶ȵ��������Ӳ���
��ش�
��1�������I2��NaHCO3��Һ�����绯��Ӧ���������к�IO-��IO![]() ���ӡ�
���ӡ�
��I2��NaHCO3��Һ��Ӧ�����¶�Ϊ40��70�棬����õļ��ȷ�ʽΪ__��
��ʵ������У�������NaI������ʹ��Ӧ���ʼӿ죬��ԭ����__��
��2�������ˮ������IO-��Ӧ�����ӷ���ʽΪ__��
��3������ಽ����Ϊ��
�ٽ������õ���pHΪ6.5��7����Һ����pHֵ��9��10����100���±���8h���õ���ҺA��
�ڽ���ҺA��pHֵ������3��4����70��80���±���4h������ҺB��
�۽���ҺB��pH������6.5��7������ҺC��
������ҺC�м������̿����Ͼ��Ⱥ���У�����10��24h���˳��ӵô�NaI��Һ��
�����٢ڢ۲����У�����pHֵʱ���μ�����Լ�Ϊ__��
A.NaOH B.HI C.NH3H2O D.�ߴ�ˮ
��4������������øĽ��ķ���Ϊ������ѹ��������������ѹ��������
������ѹ��������ѡ�õ���������Բ����ƿ������ͷ���¶ȼơ����չܡ�����ƿ֮�⣬����__��
A.ֱ�������� B.���������� C.�ձ� D.������
�ڲ�������ѹ���������ŵ�Ϊ__��
��5�����Ʊ���NaI2H2O��Ʒ��95%�Ҵ�Ϊ�ܼ������ؽᾧ������������IJ�������__��
����95%�Ҵ���____��___��___��____����Ʒ��ѡ����ţ���
�ټ�ѹ�����ᾧ ��NaI2H2O��Ʒ�ܽ� �۳��ȹ��� ����ո���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ӹ�ҵ����30%��FeCl3��Һ��ʴ���ھ�Ե���ϵ�ͭ��������ӡˢ��·�塣
��1��������Һ��Fe3�����ڵij����Լ���___��֤��Fe3�����ڵ�������____�����ƺ�Fe2������Һʱ����������Һ�м�������___��ʹ�����������γɵ�Fe3����ԭΪFe2����
��2��д��FeCl3��Һ�����ͭ������Ӧ�Ļ�ѧ����ʽ��___��
��3��ij����ʦΪ�˴�ʹ�ù��ĸ�ʴ��Һ�л���ͭ�������»��FeCl3��Һ�����������в��裺
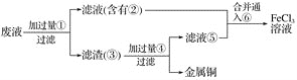
A����д������ʵ���м�������ɵ��й����ʵĻ�ѧʽ��
��___����___����___����____����____����_____��
B����д����ط�Ӧ�Ļ�ѧ����ʽ��_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���10.00mL0.1000mol��L-1HCl��0.1000mol��L-1CH3COOH�Ļ����Һ�е���0.1000mol��L-1NaOH��Һ����ҺpH�ı仯������ͼ��ʾ����֪�������£�Ka(CH3COOH)=1.75��10-5�����������������
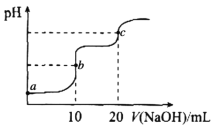
A.a����ʾ��Һ�У�CH3COOH�ĵ����ԼΪ1.75��10-2%
B.a��b��c������ʾ��Һ�У�ˮ�ĵ���̶�������c��
C.c����ʾ��Һ�У�c(Na+)>c(C1-)>c(CH3COOH>c(OH-)>c(H+)
D.����b����ʾ��Һ��![]() ��ֵ��С
��ֵ��С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ʵ��װ���������Ӧʵ�����
ѡ�� | װ��ͼ | ʵ��Ŀ�� |
A |
| �ռ������HCl |
B |
| ��ȥCO2�к��е�����HCl |
C |
| �ռ�H2��NH3��CO2��Cl2��HCl��NO��NO2������ |
D |
| ���װ�õ������� |
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������a mol FeI2����Һ�У�ͨ��x mol Cl2�����и���Ϊͨ��Cl2�����У���Һ�ڷ�����Ӧ�����ӷ���ʽ�����в���ȷ����
A. x��a��2I+Cl2��I2+2Cl
B. x=1.2a��10Fe2++14I+12Cl2��10Fe3++7I2+24Cl
C. x=1.4a��4Fe2++10I+7Cl2��4Fe3++5I2+14Cl
D. x��1.5a��2Fe2++4I+3Cl2��2Fe3++2I2+6Cl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ӷ���ʽ��������ȷ����
A.��SO2ˮ��Һ����Br2��SO2+Br2+2H2O=4H++SO42-+2Br��
B.���Ը�����غ���������ȡ����������4MnO4-+4H2O2+12H+=4Mn2++7O2��+10H2O
C.��ϡ�����ȥͭ�̣�4H����Cu2(OH)2CO3=2Cu2����CO2����3H2O
D.����Ba(HCO3)2��Һ��NaOH��Һ��Ӧ��Ba2++2HCO3��+2OH��=BaCO3��+2H2O+CO32��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
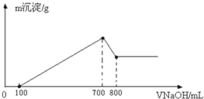
����Ŀ����1���ҹ��Ǽ��⼼���Ƚ��Ĺ��ң��챦ʯ��Al2O3�����������ڲ�������IJ��ϡ��������ӷ���ʽ��֤������һ�����������____________��___________��
��2��ȡ������������ijþ���Ͻ�ֱ����������ϡ���������������Һ�У������ı�״����H2����ֱ�Ϊ33.6L��22.4L��úϽ���þ���������ʵ���֮��Ϊ___________��
��3����һ��������þ���Ͻ��ܽ���500mL�����У���Ӧ�����Һ����μ���2mol/LNaOH��Һ�������������������Һ����Ĺ�ϵ��ͼ��ʾ������������ʵ���Ũ�ȣ����跴Ӧǰ����Һ����ı仯���Բ��ƣ�_______________�������������������ֵ_____________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�![]() �Ƚ�
�Ƚ�![]() ����ˮ�Ƴ�
����ˮ�Ƴ�![]() ��Һ��Ȼ��ȡ��
��Һ��Ȼ��ȡ��![]() ��Һ�μ�
��Һ�μ�![]() ��Һ����������������
��Һ����������������![]() ���ݲɼ��������Һ��
���ݲɼ��������Һ��![]() �仯������ͼ��ʾ(ʵ������в�����
�仯������ͼ��ʾ(ʵ������в�����![]() �Ļӷ���
�Ļӷ���![]() �ķֽ�)������˵����ȷ����
�ķֽ�)������˵����ȷ����
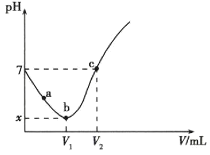
A.![]() �����У�����������ˮ�Ĺ��̣�
�����У�����������ˮ�Ĺ��̣�![]() ��ˮ�ĵ���̶Ⱦ���������
��ˮ�ĵ���̶Ⱦ���������
B.![]() ����Һ��
����Һ��![]() �ӽ���
�ӽ���![]()
C.![]() ����Һ��
����Һ��![]()
D.�μ�![]() ��Һ�����Ϊ
��Һ�����Ϊ![]() ʱ��
ʱ��![]()
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com