��������ȡ�봢��������Դ����������о��ȵ㣮
��1����֪��2H
2S��g���T2H
2��g��+S
2��g����H=169.8kJ?mol
-1�����йظ÷�Ӧ��������ȷ����
A������Ӧ���С��169.8kJ?mol
-1B���淴Ӧ���һ��С��169.8kJ?mol
-1C������Ӧ��ܲ�С��169.8kJ?mol
-1D������Ӧ��ܱ��淴Ӧ���С��169.8kJ?mol
-1��2��H
2S�ȷֽ�����ʱ������Ӧ����ͨ��һ������������ʹ����H
2Sȼ�գ���Ŀ����
��
��3��H
2O���ȷֽ�Ҳ�ɵõ�H
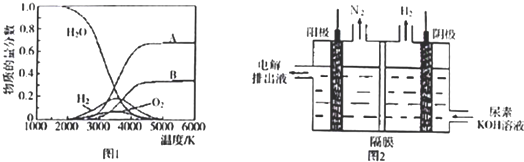
2��������ˮ�ֽ���ϵ����Ҫ���������������¶ȵĹ�ϵ����ͼ1��ʾ����4000�桫5000��ʱ���ܷ���������Щ��Ӧ
����д��ĸ����
A.2H
2O
2H
2+O
2 B��H
22H C��O
22O
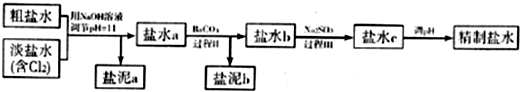
��4����ȡ��������һ�ַ����ǵ������[CO��NH
2��
2]�ļ�����Һ��װ��ʾ��ͼ��ͼ2�������и�Ĥ����ֹ����ͨ��������������Ϊ���Ե缫�����õ��װ���е���ų�Һ�е���Ҫ�ɷ���
��д��ѧʽ����
��5����֪�������ʵ�KSP��CaCO
3��10
-9��BaSO
4��1��10
-10��BaCO
3 5��10
-8��Mg��OH��
2 5.6��10
-12��
Ca��OH��
2��1.4��10
-5���ȼҵ�� ��ⱥ��ʳ��ˮҲ�ܵõ�������������õ���ˮ�辫�ƣ�ȥ����Ӱ���Ca
2+��Mg
2+��
NH
+4��SO
2-4[c��SO
2-4����c��Ca
2+��]��ij�����������£�
������a����ɳ�⣬�����е�������
��
�ڹ���I�н�NH
+4ת��ΪN
2�����ӷ���ʽ��
��
�۹���II�г�ȥ��������
��
�ܾ�����III������������ˮc��ʣ��Na
2SO
3�ĺ���С��5mg/L������ˮb��NaClO�ĺ�����7.45mg/L������10m
3��ˮb����������10%Na
2SO
3��Һ
kg����Һ����仯���Բ��ƣ�




 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д� ������ϵ�д�
������ϵ�д�
 ����ѧ--���ʽṹ�����ʡ�
����ѧ--���ʽṹ�����ʡ� ��b��CH4�� c��CH2=CHCH3��d��CH3CH2C��CH��
��b��CH4�� c��CH2=CHCH3��d��CH3CH2C��CH�� 

