
(14��)Na2S2O3��5H2O�׳ơ�������������������������Ӱ���ͻ�ԭ��������ɫ������ˮ�ľ��壬�������Ҵ�����20��C��70��Cʱ���ܽ�ȷֱ�Ϊ60.0g��212g�� Na2S2O3��5H2O��40��45��C�ۻ���48��C�ֽ⡣��֪Na2S2O3��ϡ��Һ��BaCl2��Һ����������ɡ�������Na2S2O3��5H2O��ʵ�����Ʊ����������ʵ�顣
�Ʊ������ķ�Ӧԭ����Na2SO3+S Na2S2O3
Na2S2O3
�Ʊ����������̣�
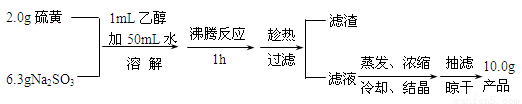
��1��ʵ�鿪ʼʱ��1mL�Ҵ���ʪ��۵������� ������ĸ����
A��������������������Ƶij�ֽӴ�
B����ֹ���������ܽ�
C��������Һ��pH
D����߲�Ʒ�Ĵ���
��2�����ȹ��˵�ԭ���� ��
��3����Һ������ֱ�������ᾧ�Ŀ���ԭ���� ��
��4�����˹�������Ҫϴ�Ӳ�Ʒ���壬����Һ�����ʺϵ��� ������ĸ����
A����ˮ�Ҵ� B������NaCl��Һ C��ˮ D����Һ
��5��Ϊ��֤��Ʒ�к���Na2SO3��Na2SO4���뽫����ʵ�鷽�����������������Լ���ϡHNO3��ϡH2SO4��ϡHCl��BaCl2��Һ������ˮ��ѡ��
��ȡ������Ʒ���ϡ��Һ�� �����ɰ�ɫ������
�� ��������δ��ȫ�ܽ⣬���д̼�����ζ������������ȷ����Ʒ�к���Na2SO3��Na2SO4���������Ƶõ�Na2S2O3��5H2O�ֲ�Ʒ��ͨ�� �����ᴿ��
17.��14�֣���1��A��2�֣���2����ֹ�¶Ƚ��Ͷ�ʹNa2S2O3��5H2O����������2�֣�
��3��ֱ�������ᾧ��ʹNa2S2O3��5H2O�ۻ��ֽ⣨2�֣�
��4��A��2�֣���5���ٵμ�����BaCl2��Һ��2�֣�
�ڹ��ˣ�������ˮϴ��������������м�������ϡ���ᣨ2�֣���6���ؽᾧ��2�֣�
��������
�����������1������S������ˮ�����ھƾ���ʵ�鿪ʼʱ��lmL�Ҵ���ʪ��ۣ�������������������ǵij�ֽӴ�����Ϊ��A����2�����¶Ƚ��ͣ�Na2S2O3���ܽ�Ȼ��С������Na2S2O3�ľ������������õ���Na2S2O3���٣���Ϊ����ֹ�¶Ƚ��Ͷ�ʹNa2S2O3������������3������Na2S2O3 40��45���ۻ���48��ֽ⣬��ֱ�������ᾧ����ʹ�����ڻ����ֽ⣬�ò���Na2S2O3?5H2O����Ϊ��ֱ�������ᾧ��ʹNa2S2O3?5H2O�ۻ��ֽ⣻��4������Na2S2O3������ˮ�������ڴ���ѡ����ˮ�Ҵ����Խ�����������Ƶ���ʧ����Ϊ��A��
��5��Ϊ��֤��Ʒ�к���Na2SO3��Na2SO4�����岽���Ǣ�ȡ������Ʒ���ϡ��Һ���μ�����BaCl2��Һ�����ɰ�ɫ���������ˣ�������ˮϴ��������������м�������ϡ���ᣬ������δ��ȫ�ܽ⣬���д̼�����ζ������������ȷ����Ʒ�к���Na2SO3��Na2SO4����6��ֱ�������ᾧ��ʹNa2S2O3?5H2O�ۻ��ֽ⣬�������Ƶõ�Na2S2O3��5H2O�ֲ�Ʒ��ͨ���ؽᾧ�ķ����ᴿ��
���㣺����Na2S2O3��5H2O��ʵ�����Ʊ����������ʵ�顣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ������ʡ������ѧ�ڵڶ���ģ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
����һ�����˵������������ص�Ԫ�أ���֪I2������NaOH��KI��Һ����Ӧ����ʽ�ֱ�Ϊ��3I2��6OH��===5I����IO��3H2O(HIO���ȶ��������绯��Ӧ)��I2��I��===I3�����廯��(IBr)��һ��±�ػ����������±�ص������Ƶ����ʣ����з�Ӧ����ʽ�в���ȷ����
A��IBr����Na2S��Һ�в�����ɫ���ǣ�IBr��S2��===I����Br����S��
B��IBr����KI��Һ�У�IBr��2I��===Br����I3��
C��IBr����NaOH��Һ�У�IBr��2OH��===I����BrO����H2O
D��IBr����AgNO3��Һ�У�3IBr��5Ag����3H2O===3AgBr����2AgI����IO3����6H��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ������ʡ������ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
������ѧ����������������������������װ�ã���ԭ�������Ƚ�����(NaAuCl4)��Һ�������е������Ƿ�Ӧ���������ʿ���(ֱ��Ϊ20 nm��60 nm)�������й�˵���д�����ǣ�
A�����ʱNaAuCl4����������Ӧ
B�������ǵĽṹ��ʽΪCH2OH(CHOH)4CHO
C�������Ǿ��л�ԭ��
D�����������ɢ��ˮ�����õķ�ɢϵ�ܲ��������ЧӦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꽭��ʡ������ʮ���ظ�����ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
FeCl3(aq)��KSCN(aq)���ʱ��������ƽ�⣺Fe3+(aq)��SCN-(aq)  Fe(SCN)2+(aq)����֪ƽ��ʱ�����ʵ���Ũ��c[Fe(SCN)2+]���¶�T�Ĺ�ϵ��ͼ��ʾ��
Fe(SCN)2+(aq)����֪ƽ��ʱ�����ʵ���Ũ��c[Fe(SCN)2+]���¶�T�Ĺ�ϵ��ͼ��ʾ��
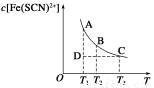
������˵����ȷ����
A��FeCl3(aq)��KSCN(aq)��Ӧ���Ȼ�ѧ��Ӧ����ʽΪ:Fe3��(aq)��SCN��(aq) Fe(SCN)2��(aq) ��H��0
Fe(SCN)2��(aq) ��H��0
B���¶�ΪT1��T2ʱ����Ӧ��ƽ�ⳣ���ֱ�ΪK1��K2����K1��K2
C����Ӧ����D��ʱ��һ���Ц���������
D��A����B����ȣ�A���c(Fe3��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꽭��ʡ������ʮ���ظ�����ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʵ�鷽���ܴﵽĿ�ĵ���
A���ð�ˮ��ϴ�Թ��ڱڸ��ŵ�����
B����NH4Cl��Һ�����Ʊ�NH4Cl����
C���Ʊ�Fe(OH)3���壬��ʢ�з�ˮ���ձ��еμ�FeCl3������Һ����ʱ�����
D������K3��Fe(CN)6����Һ����FeCl3��Һ���Ƿ����Fe2+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꽭��ʡ̩���н�����������ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ӻ��������Һ���ܴ������棬��ͨ��NO2��ᷢ����ѧ��Ӧ�����ɳ�����һ����
A��Ba2+�� Na+��Cl�C��HSO3�� B��NH4+��K+��ClO�C��S2�C
C��Cu2+��Fe2+��NO3�C��SO42�� D��Ag+��Na+��NH3��H2O��SO42�C
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�꽭��ʡ̩���н�����������ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʵ��װ����������ȷ����
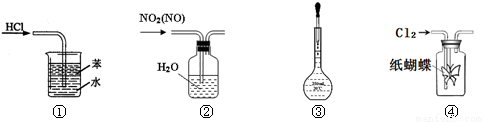
A��װ�â٣���������HCl����
B��װ�âڣ����ڳ�ȥNO2�е�NO
C��װ�âۣ�����1mol/L NaCl��Һ���ݲ���
D��װ�âܣ����е��۵⻯����Һ��ֽ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���л�ѧ������ȷ����
A��HClO�ĵ���ʽ�� |
B��������Ϊ10����ԭ�ӣ� |
C���������Ľṹ��ʽ��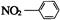 |
D��CH4���ӵ����ģ�ͣ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��˹ƥ�֣�����ʽΪC9H8O4����������֪���θ�ðҩ�����н�����ʹ���á� ����Ħ��������
| A��148g | B��148g/mol | C��180g/mol | D��146g |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com