
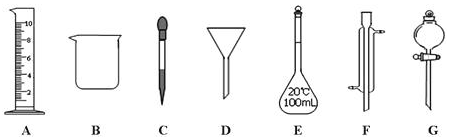
���� ��1�����������Ľṹ�ص��ж����������ƣ�
��2��GΪ��Һ©���������ڷ��뻥�����ܵ�Һ�����
��3����EΪ����ƿ��ֻ���ڳ�����ʹ�ã���ֻ������������Һ������������;��
�ڸ���0.1L��0.5mol/L=10mol/L��V����Ũ����Ũ�ȣ�������ȡҺ�壬������Ͳ�ͽ�ͷ�ιܣ�
��� �⣺��1����������ͼ�ο�֪FΪ�����ܣ��ʴ�Ϊ�������ܣ�
��2��GΪ��Һ©���������ڷ��뻥�����ܵ�Һ�����ӦΪa���ʴ�Ϊ��a��
��3����EΪ����ƿ��ֻ���ڳ�����ʹ�ã���ֻ������������Һ����ʹ��ǰҪ����Ƿ�©ˮ���ʴ�Ϊ��a��c��
��0.1L��0.5mol/L=10mol/L��V��V=0.005L����5.0mL��ȡ�ø��������ʱ����Ҫ�õ����������е���Ͳ�ͽ�ͷ�ιܣ����н�ͷ�ι����ڶ��ݣ�
�ʴ�Ϊ��5.0��C��
���� ���⿼���Ϊ�ۺϣ��漰���ʵķ����ᴿ��������ʹ�á���Һ�����Ƶ�֪ʶ�Ŀ��飬����ѧ���Ļ���ʵ�����������ʵ�����֪ʶ����Ŀ�ѶȲ���


 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��ѧ���� | ʵ��Ӧ�� | |
| A | Al2��SO4��3��С�մ�Ӧ | ��ĭ�������� |
| B | �������ξ��������� | Ư��Ư��֯�� |
| C | HF��SiO2��Ӧ | ������ڲ��������Ͽ�ʴ��� |
| D | ����ͭ������ǿ | FeCl3��ʴCu����ӡˢ��·�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 12 | B�� | 15 | C�� | 16 | D�� | 14 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
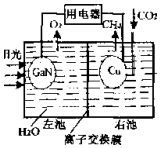 ̫���ܹ������ھ��пɿ��Ժá����������ص㣬���ںܶ����뻷���ͳ��ϣ����ѵõ��㷺Ӧ�ã������أ�GaN�����صĽṹ��ͼ��ʾ������˵������ȷ���ǣ�������
̫���ܹ������ھ��пɿ��Ժá����������ص㣬���ںܶ����뻷���ͳ��ϣ����ѵõ��㷺Ӧ�ã������أ�GaN�����صĽṹ��ͼ��ʾ������˵������ȷ���ǣ�������| A�� | ��װ��ϵͳ��ֻ������������ת�� | |
| B�� | Cu�缫�ϵĵ缫��ӦΪ��CO2+8e-+8H+�TCH4+2H2O | |
| C�� | ���ӽ���ĤΪ���ӽ���Ĥ��H+���ҳ�������� | |
| D�� | �����£���װ������1mol CH4����ʱ��GaN�缫�� 44.8LO2���ɣ�������O2���ܽ��ԣ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��״���£�11.2 L CHCl3 �к��еķ�����Ϊ 0.5NA | |
| B�� | 1mol H218O �к��е�������Ϊ 10NA | |
| C�� | 46g C2H5OH �к��еĹ��ۼ���ĿΪ 7NA | |
| D�� | 1L 1mol/L CH3COONa ��Һ�к� CH3COO-����ĿΪ NA |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com