
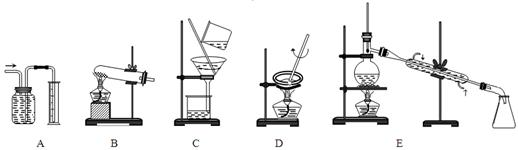
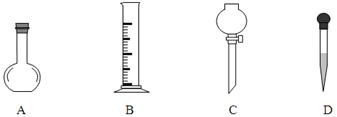
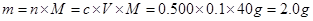 ��������450mL����������㣬��Ϊû������ݻ�������ƿ��������Һ���ƵIJ��裺һ�㣨���㣩�����ƣ������������ܣ��ܽ⣩����ת��ת�ƣ�����ϴ��ϴ�ӣ������������ݣ�����ҡ��ҡ�ȣ�����װ��װƿ��������������ǩ����Ӧ����abdcfe��
��������450mL����������㣬��Ϊû������ݻ�������ƿ��������Һ���ƵIJ��裺һ�㣨���㣩�����ƣ������������ܣ��ܽ⣩����ת��ת�ƣ�����ϴ��ϴ�ӣ������������ݣ�����ҡ��ҡ�ȣ�����װ��װƿ��������������ǩ����Ӧ����abdcfe��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A������ʱ����Һ�� | B������ʱ����ƿû�и��� |
| C��ת��ʱû��ϴ���ձ��Ͳ����� | D������ʱ����λ�÷Ŵ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢ� | B���ڢ� | C���ۢ� | D���ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
|
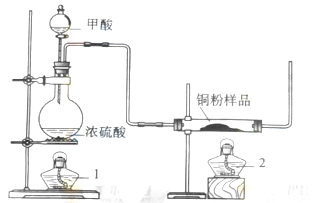 .��1���Ʊ�һ����̼�Ļ�ѧ����ʽ�� ��
.��1���Ʊ�һ����̼�Ļ�ѧ����ʽ�� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A���٢ڢܢ� | B���ڢۢܢ� | C���ڢܢ� | D���ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ȡ12.5g������CuSO4��5H2O�������500mL��Һ������ |
| B����ȡ12.5g������CuSO4��5H2O��������500mLˮ������ |
| C����ȡ7.68g��ˮ����ͭ��ĩ������480mLˮ |
| D����ȡ8.0g��ˮ����ͭ��ĩ������500mLˮ������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com