
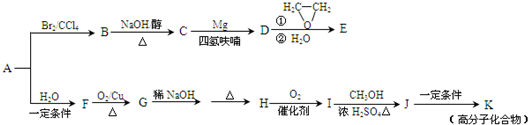
��15�֣���A��һ����Ҫ�Ļ�������ԭ�ϣ��������������Է�������Ϊ28����ͼ����AΪԭ�Ϻϳ�ҩ���м���E����֬K��·�ߡ�
��֪��I. 
II. 
��R��R����ʾ��������ԭ�ӣ�
��1��A�й����ŵĽṹ��ʽ�� ���л���B������
��2��B��C�Ļ�ѧ����ʽΪ ��B���������Ƶ�ˮ��Һ���ȷ�Ӧ���õ����л�������Ҷ��ᷴӦ���ɸ߷��ӻ����д�����ɸ߷��ӻ����ﷴӦ�Ļ�ѧ����ʽ
��3��E�ķ���ʽΪC4H8O�����й���E��˵����ȷ���� ������ĸ��ţ���
a. ��������Ʒ�Ӧ b. ������4��̼ԭ��һ����ƽ��
c. һ�������£�����Ũ�����ᷴӦ d. ��CH2=CHCH2OCH2CH3��Ϊͬϵ��
��4��G��H�漰���ķ�Ӧ������ ��
��5��I�ķ���ʽΪC4H6O2����ṹ��ʽΪ ��
��6��J��K�Ļ�ѧ����ʽΪ ��
��7��д����E������ͬ�����ŵ�����ͬ���칹��Ľṹ��ʽ��
��������˳���칹�������ǡ�OH����˫��̼�ϵĽṹ����
��1��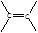 1,2-��������
1,2-��������
��2��BrCH2CH2Br + NaOH 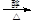 CH2="CHBr" + NaBr + H2O
CH2="CHBr" + NaBr + H2O
nHOOCCOOH+nHOCH2CH2OH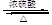
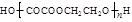 +(2n��1)H2O
+(2n��1)H2O
��3��ac
��4���ӳɷ�Ӧ����ȥ��Ӧ
��5��CH3CH=CHCOOH
��6��n CH3CH=CHCOOCH3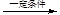
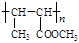
��7��CH3CH=CHCH2OH 

���������������Է�������Ϊ28����ֻ����ΪC2H4��AΪCH2=CH2����BΪCH2BrCH2Br��B��C�ķ�ӦΪ��ȥ��Ӧ������ϢI��C��D��E��Ӧ������֪B��C��ȥһ����HBr��CΪCH2=CHBr��DΪCH2=CHMgBr��EΪCH2=CHCH2CH2OH��
CH2=CH2��H2O�����ӳɷ�Ӧ����CH3CH2OH��F����GΪCH3CHO��ģ����ϢII����֪HΪCH3CH=CHCHO��IΪCH3CH=CHCOOH��JΪCH3CH=CHCOOCH3��KΪ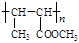 ��
��
��1����ϩ�й�����Ϊ̼̼˫�����ṹ��ʽΪ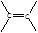 ��B������Ϊ1��2-�������顣
��B������Ϊ1��2-�������顣
��2��B����CΪ��ȥ��Ӧ������ʽΪBrCH2CH2Br + NaOH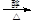 CH2="CHBr" + NaBr + H2O��BrCH2CH2Br����������ˮ��Һ����ˮ�ⷴӦ����HOCH2CH2OH�������Ҷ����ܷ������۷�Ӧ����Ӧ����ʽΪnHOOCCOOH+nHOCH2CH2OH
CH2="CHBr" + NaBr + H2O��BrCH2CH2Br����������ˮ��Һ����ˮ�ⷴӦ����HOCH2CH2OH�������Ҷ����ܷ������۷�Ӧ����Ӧ����ʽΪnHOOCCOOH+nHOCH2CH2OH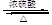
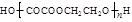 +(2n��1)H2O��
+(2n��1)H2O��
��3��EΪCH2=CHCH2CH2OH�������к����ǻ�������Na����������a��ȷ��CH2=CHCH2����3��̼ԭ��һ����ƽ�棬��CH2OH��̼ԭ�ӿ��ܹ��棬Ҳ���ܲ����棬b����CH2=CHCH2CH2OH���������ᷴӦ����CH2=CHCH2CH2Br��H2O��c��ȷ��ͬϵ��ָ�ṹ���ƣ�����������n��CH2�Ļ����CH2=CHCH2CH2OH���ڴ��࣬CH2=CHCH2OCH2CH3�������࣬����Ϊͬ���칹�壬d����
��4��G��H�漰�ķ�ӦΪ�ȼӳɺ���ȥ��
��5��IΪCH3CH=CHCOOH��
��6��J��KΪ�Ӿ۷�Ӧ����Ӧ����ʽΪn CH3CH=CHCOOCH3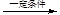
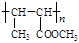 ��
��
��7��EΪCH2=CHCH2CH2OH����E������ͬ�����ŵ�ͬ���칹�廹��3�֣��ṹ��ʽΪ��
CH3CH=CHCH2OH �� ��
�� ��
��
���㣺�л���Ӧ���� �л������ɡ��ṹ������ �л���ѧ��Ӧ ͬ���칹��
������������ʼԭ�ϼ��м�����ƶ�ʱҪ��������Ϣ��������ܻ�ʹ˼ά���衣


 ������ȫ��������ϵ�д�
������ȫ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�




| �� |
| �� |
| �� |
| �� |




�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



| �� |
| �� |
| �� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| �� |
| �� |
| �� |
| �� |
| һ������ |
 +2��n-1��H2O
+2��n-1��H2O| һ������ |
 +2��n-1��H2O
+2��n-1��H2O



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ�����и���3��ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ������
��A��һ����Ҫ�Ļ�������ԭ�ϣ��������������Է�������Ϊ28����ͼ����AΪԭ�Ϻϳ�ҩ���м���E����֬K��·�ߡ�

��֪��I. 
II. 
��R��R����ʾ��������ԭ�ӣ�
��1��A�й������� ��E�Ľṹ��ʽ�� ��
��2��B��C�Ļ�ѧ����ʽΪ ��
��3��G��H�漰���ķ�Ӧ������ ��H��I�漰���ķ�Ӧ������ ��
��4��д����E������ͬ�����ŵ�����ͬ���칹��Ľṹ��ʽ��д�����ּ��ɣ���
��������˳���칹�������ǡ�OH����˫��̼�ϵĽṹ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com