
��ϡH2SO4��NaOH��Һ�ͷ���Ϊԭ����ȡAl(OH)3���ס��ҡ�����λͬѧ���Ʊ�;�����£���Դ˻ش����⣺
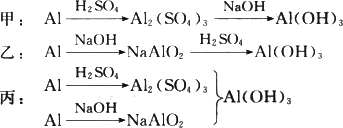
(1)�����������У�������һ���Ļ�ѧ�仯���漰������������Һ�з�����Ӧ��д���û�ѧ��Ӧ�����ӷ���ʽ��________��
(2)�ӻ��ͬ����IJ�Ʒ��ԭ���������ٵĽǶ��������������������������________��������Ϊ��������ķ����У���ȥԭ�����������������⣬���з����������������(��ѡһ������̸һ����Ҫ����)________��
(3)������Ϊ����ķ����У�Ϊ�˴ﵽʹһ�������ķ���������õ�Ŀ�ģ��ڷ���ʵʩ֮ǰ����Ҫ���Ķ���ʵ����________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ������Ԫ��A��B��C��D��E��F��ԭ����������������֪����A��Eͬ���壬E�ĵ�����D2��Ӧ������E2D��E2D2���ֹ��壻��F�ĵ�����D2��ȼ�յIJ����ʹƷ����Һ��ɫ��B�ĵ�����D2��ȼ�տ�����BD��BD2�������壻��CA4++DA-=CA3��+A2D�����ַ�Ӧ��������ĵ���������E+��ȣ���ش��������⣺
������Ԫ��A��B��C��D��E��F��ԭ����������������֪����A��Eͬ���壬E�ĵ�����D2��Ӧ������E2D��E2D2���ֹ��壻��F�ĵ�����D2��ȼ�յIJ����ʹƷ����Һ��ɫ��B�ĵ�����D2��ȼ�տ�����BD��BD2�������壻��CA4++DA-=CA3��+A2D�����ַ�Ӧ��������ĵ���������E+��ȣ���ش��������⣺



| ||
| 2 |
| 3 |
| ||
| ||
| 2 |
| 3 |
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



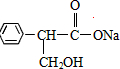
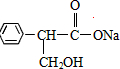
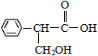

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 50w |
| 9av |
| 50w |
| 9av |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㶫ʡ2009�������ѧ������У�¿������࣭ʵ��̽��(1) ���ͣ�058
��֪SiO2��SO2��CO2���������������ѧ���ʾ���һ���������ԣ�Mg��Na�Ļ�ѧ����Ҳ����һ����������(��ʾ��2Mg��CO2![]() 2MgO��C)
2MgO��C)
������ͼ��ʾ��װ�ý���Mg��SO2��ʵ�飺
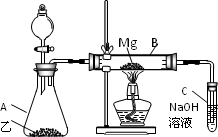
(1)ѡ����ȡSO2�ĺ����Լ�________��
��10����H2SO4��Һ��
��80����H2SO4��Һ��
��Na2SO3���壻
��CaSO3����
(2)д��װ��B�з�������Ҫ��Ӧ�Ļ�ѧ����ʽ��________
(3)����Ϊ��װ���Ƿ��в���֮����________������У����г����㣺__________��
��ij�о���ѧϰС������ˡ�ʵ������Si�����о��������Կα�Ϊ�������������Ϻ�õ����¿ɹ��ο�����Ϣ��
�ٹ�ҵ���ڸ���ʱ��C��ԭSiO2���Ƶ�Si��
��Mg�ڸ��µ������¼�����SiO2��Ӧ��
�۽����軯����ϡH2SO4��Ӧ���������κ�SiH4��
��Si��SiO2������ϡH2SO4��Ӧ��
��SiH4�ڿ�������ȼ��
�������о������м����š�����ѡ�ú��ʵ����������˵������³�ַ�Ӧ����������ϡH2SO4�ܽ������Ȼ����ˡ�ϴ�ӡ������������������ϡH2SO4�ܽ�������ʱ�������б������ͻ������Ҳֻ��Ԥ��ֵ��63�����ҡ���
(4)��С�顰ʵ�����ơ�Si���Ļ�ѧ����ʽ��________
(5)����ơ���ϡH2SO4�ܽ�������ʱ�������б������ͻ���ԭ����________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㶫ʡ2009�������ѧ������У�¿������࣭̼�輰�仯���� ���ͣ�058
��֪SiO2��SO2��CO2���������������ѧ���ʾ���һ���������ԣ�Mg��Na�Ļ�ѧ����Ҳ����һ����������(��ʾ��2Mg��CO2![]() 2MgO��C)
2MgO��C)
������ͼ��ʾ��װ�ý���Mg��SO2��ʵ�飺
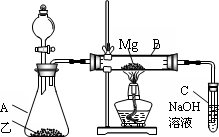
(1)ѡ����ȡSO2�ĺ����Լ�________��
��10����H2SO4��Һ��
��80����H2SO4��Һ��
��Na2SO3���壻
��CaSO3����
(2)д��װ��B�з�������Ҫ��Ӧ�Ļ�ѧ����ʽ��________________
(3)����Ϊ��װ���Ƿ��в���֮����________������У����г����㣺__________��
��ij�о���ѧϰС������ˡ�ʵ������Si�����о��������Կα�Ϊ�������������Ϻ�õ����¿ɹ��ο�����Ϣ��
�ٹ�ҵ���ڸ���ʱ��C��ԭSiO2���Ƶ�Si��
��Mg�ڸ��µ������¼�����SiO2��Ӧ��
�۽����軯����ϡH2SO4��Ӧ���������κ�SiH4��
��Si��SiO2������ϡH2SO4��Ӧ��
��SiH4�ڿ�������ȼ��
�������о������м����š�����ѡ�ú��ʵ����������˵������³�ַ�Ӧ����������ϡH2SO4�ܽ������Ȼ����ˡ�ϴ�ӡ������������������ϡH2SO4�ܽ�������ʱ�������б������ͻ������Ҳֻ��Ԥ��ֵ��63�����ҡ���
(4)��С�顰ʵ�����ơ�Si���Ļ�ѧ����ʽ��________
(5)����ơ���ϡH2SO4�ܽ�������ʱ�������б������ͻ���ԭ����________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com