
| ×å ÖÜĘŚ |
IA | IIA | IIIA | VIA | VA | VIA | VIIA | 0 |
| 2 | ¢Ž | ¢ß | ||||||
| 3 | ¢Ł | ¢Ū | ¢Ż | ¢ą | ¢ā | |||
| 4 | ¢Ś | ¢Ü | ¢į |
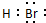
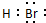
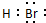 £¬Ė®Óė¼Ų·“Ӧɜ³ÉĒāŃõ»Æ¼ŲŗĶĒāĘų£¬Ęä»Æѧ·“Ó¦·½³ĢŹ½ĪŖ2K+2H2OØT2KOH+H2”ü£¬Ąė×Ó·“Ó¦ĪŖ2K+2H2OØT2K++2OH-+H2”ü£¬
£¬Ė®Óė¼Ų·“Ӧɜ³ÉĒāŃõ»Æ¼ŲŗĶĒāĘų£¬Ęä»Æѧ·“Ó¦·½³ĢŹ½ĪŖ2K+2H2OØT2KOH+H2”ü£¬Ąė×Ó·“Ó¦ĪŖ2K+2H2OØT2K++2OH-+H2”ü£¬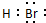 £»2K+2H2OØT2K++2OH-+H2”ü£»
£»2K+2H2OØT2K++2OH-+H2”ü£»


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| H | He | ||||||
| A | B | ||||||
| C | D | E | F | G |
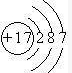



²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
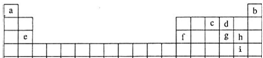
| ||
| ”÷ |
| ||
| ”÷ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø2010?ĮijĒÄ£Äā£©Čē±ķŹĒŌŖĖŲÖÜĘŚ±ķµÄŅ»²æ·Ö£®X”¢Y”¢Z¾łĪŖ¶ĢÖÜĘŚŌŖĖŲ£¬X”¢ZµÄÖŹ×ÓŹżÖ®ŗĶĪŖ23£®ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©
|
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
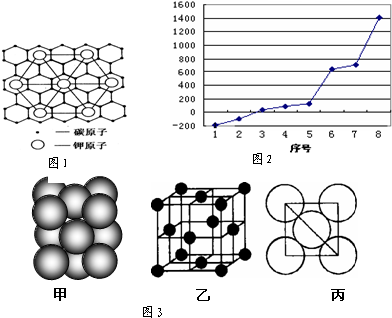
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ×å ÖÜĘŚ |
IA | IIA | IIIA | IVA | VA | VIA | VIIA |
| 1 | A | ||||||
| 2 | B | C | D | E | J | K | L |
| 3 | F | G | H | I | |||
| M |
 ĖüµÄæÕ¼äĄūÓĆĀŹĪŖ
ĖüµÄæÕ¼äĄūÓĆĀŹĪŖ²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com