

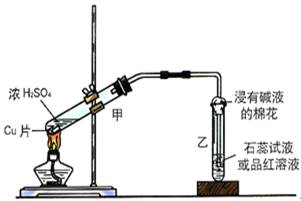
| ŠņŗÅ | Ń”ÓƵÄŅĒĘ÷(Ģī×ÖÄø) | ¼ÓČėµÄŹŌ¼Į | ×÷ÓĆ |
| ¢Ł | B | ÅØĮņĖį”¢ĪŽĖ®ŅŅ“¼ | ·“Ó¦Ę÷ |
| ¢Ś | | | |
| ¢Ū | C | Ę·ŗģČÜŅŗ | |
| ¢Ü | C | | ĪüŹÕSO2 |
| ¢Ż | C | Ę·ŗģČÜŅŗ | |
| ¢Ž | C | | ¼ģ³öŅŅĻ© |
| ¢ß | C | | ¼ģ³öCO2 |
 CH2£½CH2”ü£«H2O(2·Ö)
CH2£½CH2”ü£«H2O(2·Ö) CO2”ü£«2SO2”ü£«2H2O»ņC2H5OH£«6H2SO4(ÅØ)
CO2”ü£«2SO2”ü£«2H2O»ņC2H5OH£«6H2SO4(ÅØ) 2CO2”ü£«6SO2”ü£«9H2O(2·Ö)
2CO2”ü£«6SO2”ü£«9H2O(2·Ö)| ŠņŗÅ | Ń”ÓƵÄŅĒĘ÷(Ģī×ÖÄø) | ¼ÓČėµÄŹŌ¼Į | ×÷ÓĆ |
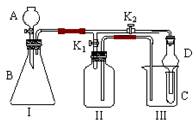
| ¢Ś | A | ĪŽĖ®ĮņĖįĶ | ¼ģ³öĖ®ÕōĘų |
| ¢Ū | | | ¼ģ³öSO2 |
| ¢Ü | | FeCl3ČÜŅŗ | |
| ¢Ż | | | ¼ģŃéSO2ŹĒ·ńĪüŹÕĶźČ« |
| ¢Ž | | äåĖ® | |
| ¢ß | | ³ĪĒåŹÆ»ŅĖ® | |


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®½«25g CuSO4”¤5H2OČÜÓŚ1LĖ®ÖŠ£¬æÉÅäÖĘ³É0£®1 mol”¤L-1CuSO4ČÜŅŗ |
| B£®ÓĆŃĪĪö·Ø³żČ„ĒāŃõ»ÆĢś½ŗĢåÖŠ»ģÓŠµÄĀČĄė×Ó |
| C£®ÓĆĻņĻĀÅÅæÕĘų·ØŹÕ¼ÆNH”££¬²¢ÓĆŹŖČóµÄĄ¶É«ŹÆČļŹŌÖ½¼ģŃéNH”£ŹĒ·ńŹÕ¼ÆĀśĮĖ |
| D£®ÖĘŅŅČ²Ź±£¬ÓƱ„ŗĶŹ³ŃĪĖ®“śĢęĖ®ŹĒĪŖĮĖ¼õ»ŗµēŹÆÓėĖ®µÄ·“Ó¦ĖŁĀŹ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®±½µÄĻõ»ÆÖĘĻõ»ł±½ | B£®ŅŅĖįŅŅõ„µÄĖ®½ā |
| C£®ÓĆŅŅ“¼ÖĘŅŅĆŃ | D£®ĀóŃæĢĒÓėŅų°±ČÜŅŗµÄ·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
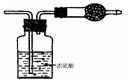
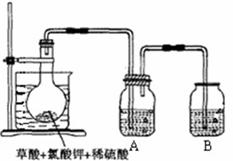
| A£®øÉŌļ¹ÜÄŚ¹ĢĢåĪŖ¼īŹÆ»Ņ | B£®ŌĘųĢåÖŠŅ»¶ØÓŠ NO ŗĶ O2 |
| C£®ŌĘųĢåÖŠŅ»¶ØÓŠNH3”¢NO ”¢CO2 | D£®ŌĘųĢåÖŠÓŠ HC1”¢ Br2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
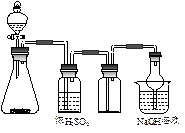
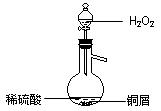
| A£®µēŹÆŗĶĖ® |
| B£®MnO2ŗĶÅØŃĪĖį |
| C£®CuʬŗĶÅØĻõĖį |
| D£®Na2SO3ŗĶÅØĮņĖį |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com