
| 4.48L |
| 22.4L/mol |
| 19g |
| 0.2mol |
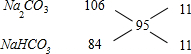
| 0.3mol |
| 0.2L |



| Дкј¶ | ёЯЦРҝОіМ | Дкј¶ | іхЦРҝОіМ |
| ёЯТ» | ёЯТ»Гв·СҝОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СҝОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СҝОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СҝОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СҝОіМНЖјцЈЎ | іхИэ | іхИэГв·СҝОіМНЖјцЈЎ |
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈәБЙДюКЎҙуБ¬¶юК®ЛДЦР2012ҪмёЯИэЙПС§ЖЪЖЪЦРҝјКФ»ҜС§КФМв МвРНЈә022
ҙўЗвДЙГЧМј№ЬөДСРЦЖіЙ№ҰМеПЦБЛҝЖјјөДҪшІҪЈ®УГөз»Ў·ЁәПіЙөДДЙГЧМј№ЬіЈ°йУРҙуБҝөДФУЦКМјДЙГЧҝЕБЈЈ¬ХвЦЦМјДЙГЧҝЕБЈҝЙУГСх»ҜЖш»Ҝ·ЁМбҙҝЈ¬·ҙУҰЦРөД·ҙУҰОпәНЙъіЙОпУРCЎўCO2ЎўH2SO4ЎўKCr2O7ЎўCr2(SO4)2ЎўH2OЖЯЦЦОпЦКЈ®
(1)ИфҪ«МјДЙГЧҝЕБЈ·ЦЙўөҪТ»¶ЁИЬјБЦРЈ¬РОіЙОИ¶ЁөД·ЦЙўПөЈ¬ЛщҫЯөДРФЦКУР________Ј®
ўЩ¶Ўҙп¶ыР§УҰ
ўЪјУИлұҘәН(NH4)2SO4ИЬТәІъЙъҫЫіБ
ўЫҝЙНЁ№э°лНёДӨ
(2)ЗлУГЙПКцОпЦКМоҝХЈ¬ЕдЖҪ»ҜС§·ҪіМКҪЈ¬ІўұкіцөзЧУЧӘТЖөД·ҪПтәНКэДҝ________C+________Ј«________H2SO4Ўъ________K2SO4Ј«________Ј«________Cr2(SO4)3Ј«________H2O
(3)Ҫ«ұкЧјЧҙҝцПВ4.48 LІъЙъөДЖшМеНЁИлККБҝөДNaOHИЬТәЦРід·Ц·ҙУҰәуЈ¬ИЬТәЦРЙъіЙСОөДЦКБҝОӘ19.0 gЈ®
(ўс)ИфТӘК№ЙъіЙөДСОөДЦКБҝұдОӘ25.2 gЈ¬ФтУҰјМРшПтИЬТәЦРНЁИлёГЖшМе________gЈ®
(ўт)ПтЙъіЙөД19.0 gөДСОИЬТәЦРјУИлТ»¶ЁБҝДіОпЦКЈ¬ід·Ц·ҙУҰәуЈ¬јхС№өНОВХф·ўөГөҪҙҝҫ»өД21.2 gЎЎNa2CO3№ММеЈ®ФтЈә
ўЩИфЦ»ДЬјУИл0.05 molДіОпЦКЈ¬ФтјУИлөДОпЦКҝЙТФКЗ________»т________Ј®
ўЪИфЦ»ДЬјУИл0.10 molДіОпЦКЈ¬ФтјУИлөДОпЦКҝЙТФКЗ________»т________Ј®
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈәҪӯОчКЎ°ЧрШЦЮЦРС§2012ҪмёЯИэөЪТ»ҙОФВҝј»ҜС§КФМв МвРНЈә058
Ҫ«ұкЧјЧҙҝцПВ4.48 LөДCO2НЁИлККБҝөДNaOHИЬТәЦРід·Ц·ҙУҰәуЈ¬ИЬТәЦРЙъіЙСОөДЦКБҝОӘ19.0 gЈ®
(1)ИфТӘК№ЙъіЙөДСОөДЦКБҝұдОӘ25.2 gЈ¬ФтУҰјМРшПтИЬТәЦРНЁИлCO2________gЈ®(РҙіцјЖЛг№эіМ)
(2)ПтЙъіЙөД19.0 gөДСОИЬТәЦРјУИлТ»¶ЁБҝДіОпЦКЈ¬ід·Ц·ҙУҰәуЈ¬јхС№өНОВХф·ўөГөҪҙҝҫ»өД21.2 gЎЎNa2CO3№ММеЈ®ФтЈә
ўЩИфЦ»ДЬјУИл0.05 molДіОпЦКЈ¬ФтјУИлөДОпЦКҝЙТФКЗ________»т________Ј®
ўЪИфЦ»ДЬјУИл0.10 molДіОпЦКЈ¬ФтјУИлөДОпЦКҝЙТФКЗ________»т________Ј®
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
A.3.8 g B.5.6 g C.4.8 g D.6.0 g
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
Ҫ«ұкЧјЧҙҝцПВ4.48 LөДCO2НЁИлККБҝөДNaOHИЬТәід·Ц·ҙУҰәуЈ¬ИЬТәЦРЙъіЙСОөДЦКБҝОӘ19.0 gЎЈ
ЈЁ1Ј©ИфТӘК№ЙъіЙөДСОөДЦКБҝұдОӘ25.2 gЈ¬УҰјМРшПтИЬТәЦРНЁИлұкЧјЧҙҝцПВCO2 gЎЈ
ЈЁ2Ј©ПтЙъіЙөД19.0 gөДСОИЬТәЦРјУИлТ»¶ЁБҝДіОпЦКЈ¬ід·Ц·ҙУҰәуЈ¬јхС№өНОВХф·ўөГөҪҙҝҫ»өД21.2 g Na2CO3№ММеЎЈФтЈә
ўЩИфЦ»ДЬјУИл0.05 molДіОпЦКЈ¬ФтјУИлөДОпЦКҝЙТФКЗ »т ЎЈ
ўЪИфЦ»ДЬјУИл0.10 molДіОпЦКЈ¬ФтјУИлөДОпЦКҝЙТФКЗ »т ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
Ҫ«ұкЧјЧҙҝцПВ4.48 LөДCO2НЁИлККБҝөДNaOHИЬТәід·Ц·ҙУҰәуЈ¬ИЬТәЦРЙъіЙСОөДЦКБҝОӘ19.0 gЎЈ
ЈЁ1Ј©ИфТӘК№ЙъіЙөДСОөДЦКБҝұдОӘ25.2 gЈ¬УҰјМРшПтИЬТәЦРНЁИлұкЧјЧҙҝцПВCO2 gЎЈ
ЈЁ2Ј©ПтЙъіЙөД19.0 gөДСОИЬТәЦРјУИлТ»¶ЁБҝДіОпЦКЈ¬ід·Ц·ҙУҰәуЈ¬јхС№өНОВХф·ўөГөҪҙҝҫ»өД21.2 g Na2CO3№ММеЎЈФтЈә
ўЩИфЦ»ДЬјУИл0.05 molДіОпЦКЈ¬ФтјУИлөДОпЦКҝЙТФКЗ »т ЎЈ
ўЪИфЦ»ДЬјУИл0.10 molДіОпЦКЈ¬ФтјУИлөДОпЦКҝЙТФКЗ »т ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
°Щ¶ИЦВРЕ - Б·П°ІбБРұн - КФМвБРұн
әюұұКЎ»ҘБӘНшОҘ·ЁәНІ»БјРЕПўҫЩұЁЖҪМЁ | НшЙПУРәҰРЕПўҫЩұЁЧЁЗш | өзРЕХ©ЖӯҫЩұЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРәҰРЕПўҫЩұЁЧЁЗш | ЙжЖуЗЦИЁҫЩұЁЧЁЗш
ОҘ·ЁәНІ»БјРЕПўҫЩұЁөз»°Јә027-86699610 ҫЩұЁУКПдЈә58377363@163.com