
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2008?����ģ�⣩����ʵ�������ȷ���ܴﵽԤ��Ŀ���ǣ�������
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ��Ϊ�ش�������ѧ2012�������ѧ��������ѧ���� ���ͣ�058
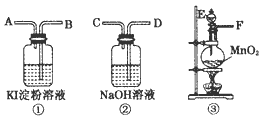
Ϊ�˱Ƚ��Ⱥ͵ⵥ�������Ե�ǿ����������ȡ��������������ͨ��⻯�ص�����Һ�У���ͼ�DZ�ʵ�������������ҩƷ��ͼ��A��B��C��D��E��F�ֱ��ʾ�������ܻ������ӿڣ�

��ش��������⣺
(1)������������ң�ʵ��ǰ������������װ�õ���ȷ����˳����(����ӿڵ���ĸ����)��D��E��������ƿ��________��________��________��________��
(2)д���Ʊ�Cl2�ķ�Ӧ����ʽ��________��
ijѧ���ú�0.4 mol��HCl��Ũ���������MnO2��Ӧ��Cl2�õ���Cl2��С��2.24 L(�����)Ϊʲô��
________
(3)ʵ����ɺ�װ�â��е���ҺΪ________ɫ���������еμ�����NaOH��Һ��ɫ�Ƿ���ȥ��________(��ǡ����ǡ�)��װ�â���NaOH��Һ�������ǣ�________��
(4)��ҵ�ϣ�����Cl2�Ʊ�Ư�ۣ���д�Ʊ�Ư�۵����ӷ�Ӧ����ʽ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�찲��ʡ��Ϊ�ش�������ѧ������ѧ��������ѧ�Ծ� ���ͣ�ʵ����
��10�֣�Ϊ�˱Ƚ��Ⱥ͵ⵥ�������Ե�ǿ����������ȡ��������������ͨ��⻯�ص�����Һ�С���ͼ�DZ�ʵ�������������ҩƷ��ͼ��A.B.C.D.E.F�ֱ��ʾ�������ܻ������ӿڡ�
��ش��������⣺
��1��������������ҡ�ʵ��ǰ������������װ�õ���ȷ����˳���ǣ�����ӿڵ���ĸ���ţ���D��E��������ƿ��_____________��_____________��_____________��_____________��
��2��д���Ʊ� �ķ�Ӧ����ʽ��______ ��
�ķ�Ӧ����ʽ��______ ��
ijѧ���ú�0.4molHCl��Ũ���������MnO2��Ӧ�� �õ���
�õ��� ��С��
��С��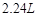 ������£�Ϊʲô��___________________
������£�Ϊʲô��___________________
��3��ʵ����ɺ�װ��1�е���ҺΪ_____________ɫ���������еμ����� ��Һ��ɫ�Ƿ���ȥ��_____________����ǡ����ǡ�����װ�â���NaOH��Һ�������ǣ�______ .
��Һ��ɫ�Ƿ���ȥ��_____________����ǡ����ǡ�����װ�â���NaOH��Һ�������ǣ�______ .
(4)��ҵ�ϣ����� �Ʊ�Ư�ۣ���д�Ʊ�Ư�۵����ӷ�Ӧ����ʽ��
�Ʊ�Ư�ۣ���д�Ʊ�Ư�۵����ӷ�Ӧ����ʽ��
____________ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com