
��5�֣���1 molI2(g)��2 mol H2(g)����2L�ܱ������У���һ�� �¶��·�����Ӧ��
�¶��·�����Ӧ��
I2(g)��H2(g) 2HI(g) DH<0�����ﵽƽ�⡣HI���������j(HI) ��ʱ��ı仯������(II)��ʾ��
2HI(g) DH<0�����ﵽƽ�⡣HI���������j(HI) ��ʱ��ı仯������(II)��ʾ��
��1���ﵽƽ���I2(g)�����ʵ���Ũ��Ϊ ��
��2�����ı䷴Ӧ��������ij������j(HI)�ı仯������(��)��ʾ��������������� ������������������ţ���
�ٺ��������£������¶�
�ں��������£������¶�
�ۺ��������£���С��Ӧ�������
�ܺ��������£�����Ӧ�������
�ݺ��¡����������£������ʵ��Ĵ���

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �ı䷴Ӧ���� | ��Ӧ���ʱ仯 | ƽ���ƶ����� |
| ��1�������¶� | ||
| ��2��������� | ||
| ��3����������H2 | ||
| ��4��������������� | ||
| ��5�������ݻ����䣬ͨ������ | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
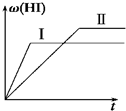 ��1mol H2��g����2molI2��g������2L�ܱ������У���һ���¶��·�����Ӧ��H2��g��+I2��g��?
��1mol H2��g����2molI2��g������2L�ܱ������У���һ���¶��·�����Ӧ��H2��g��+I2��g��?�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com