
��14��)��1����2L���ܱ������з���4molN2O5���������·�Ӧ��2N2O5(g)  4NO2(g)+ O2(g)����Ӧ5min���N2O5ת����20%���ԣ�NO2)Ϊ
��5minʱ��N2O5ռ���������������� ��
4NO2(g)+ O2(g)����Ӧ5min���N2O5ת����20%���ԣ�NO2)Ϊ
��5minʱ��N2O5ռ���������������� ��
�Ķ����ϣ��ش�(2)��(3)С��
пͭԭ����û�ͼ�ķ�ʽ����ͼ����ʾ�ܲ����㣬�������õ��ͼʽ����ʽ����Zn|ZnSO4(1mol/L)||CuSO4��1mol/L��|Cu ��ʽ�У�����������Ӧ�ĸ���д����ߣ�������ԭ��Ӧ������д���ұߡ���ʵ���ߡ�|����ʾ�缫����Һ֮��Ľ��棬��˫ʵ���ߡ�||����ʾ���š�

��2�����������оٵĵ���У�пƬ�Ϸ����ĵ缫��Ӧʽ�� ��
��3������һ��أ���ͼʽ����ʽΪCu|CuSO4(1mol/L)||Fe2(SO4)3��0.5mol/L��|C���õ���У������ĵ缫��Ӧʽ�� �������ĵ缫��Ӧʽ�� ��
��4��д��֧��ֻ��һ���һ���ʽ����С�������Ľṹ��ʽ
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ӱ�ʡ��ˮ��ѧ��һ��ѧ��һ�����Ի�ѧ�Ծ����������� ���ͣ������
����14�֣�
��1�����в����������_________������ţ�
A. ����������м�������ʵ��ʱ������ʯ������
B. Ũ�����մ��Ƥ���ϣ�Ѹ����NaOH��Һ��ϴ������ˮϴ��
C. ����ʱ�����¶ȼ�ˮ�������Һ�����¡�
D. ��������ζʱ�������������������ȶ���ʹ��������Ʈ��ǿ��С�
E. ʹ�÷�Һ©��������ƿʱ�����ȼ���Ƿ�©Һ��
(2) ���ý�屨���˶�����������ϴʱ������ʹ�á�����顱����Ҫ�ɷ������ᣩ�롰84
����Һ������Ч�ɷ���NaClO�����������ж����¼�����������ѧ��������ԭ��Ӧ֪ʶ����
���ӷ���ʽ��ʾ����������ԭ��
(3) ��֪��ƫ��������Һ�м���ϡ�����������������ɡ�ijͬѧ������ѧ֪ʶ���������ʹ�ø÷�����ȡA1(OH)3������Ϊ ���������ӷ���ʽ��ʾ���������ٳ�һ�����Ȼ�����Һ��ȡA1(OH)3�ĺ����������÷�Ӧ�����ӷ���ʽΪ ��
(4)���йر�����Ŀǰ����ұ�������ȸߴ�99��9999�����������й��ڴ����������У���ȷ���� ������ĸ����
A��Ӳ�ȱȸ�С���۵�ȸָ� B�����������ᷴӦ
C���벻��ֳɷ���ͬ D�������Ũ�����жۻ�
(5)���ˮ����εμӱ���FeCl3��Һ����Һ������ĺ��ɫʱֹͣ���ȣ����÷�ɢϵ�ķ�ɢ�ʴ�С��Χ�� ��
(6)�ϳɰ���ҵ�����г�������ý������������Ҫ�ɷ���FeO��Fe2O3����֪ij����ý�У����������ӵ����ʵ���֮��Ϊ4��5������Fe2+��Fe3+���ʵ���֮��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʦ���и߶�12�½μ�⻯ѧ�Ծ� ���ͣ������
(14��) ���ྻú�������о����������൱�ձ飬������Աͨ�������ú������¯�н�����������ˮ�����ķ�����������������ֵ�ߴ�122500~16000 kJ��m-3��ú̿��������Ҫ�ɷ���CO��H2��CO��H2����Ϊ��Դ�ͻ���ԭ�ϣ�Ӧ��ʮ�ֹ㷺��
��1����֪��
C(s)+O2(g)=CO2(g)��������H1����393.5 kJ��mol-1��������
2H2(g)+O2(g)=2H2O(g)������H2����483.6 kJ��mol-1���� ��
C(s)+H2O(g)=CO(g)+H2(g)����H3����131.3 kJ��mol-1�� ��
��ӦCO(g)+H2(g) +O2(g)= H2O(g)+CO2(g)����H= ������������kJ��mol-1����״���µ�ú̿����CO��H2��33.6 L��������ȫ��Ӧ����CO2��H2O����Ӧ������ת����������mol e-��
��2�������¶�650���������ȼ�ϵ�أ�����ú̿����CO��H2��������ȼ����������CO2�Ļ��������������Ӧ����һ��������Li2CO3��Na2CO3���۵�����������ʣ��Խ�������ȼ�ϼ���Ϊ�����Ƴɵġ������ĵ缫��ӦʽΪ��CO+H2-4e-+2CO32-=3CO2+H2O����õ�ص�������ӦʽΪ���� ��������
��3���ܱ������г���10 mol CO��20 mol H2���ڴ��������·�Ӧ���ɼ״���CO(g)+2H2(g)  CH3OH(g)��CO��ƽ��ת���ʣ��������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
CH3OH(g)��CO��ƽ��ת���ʣ��������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
����A��B�����ʾ��ijʱ�̴ﵽ��ƽ��״̬����ʱ��A��ʱ���������ΪVAL������¶��µ�ƽ�ⳣ��K=���� ��������A��B����ʱ���������ʵ����ʵ���֮��Ϊn(A)����n(B)��=���� ��������
����A��C���㶼��ʾ�ﵽ��ƽ��״̬�����Է�Ӧ��ʼ����ƽ��״̬�����ʱ��tA���� ������tC������ڡ�����С�ڡ����ڡ�����
���ڲ��ı䷴Ӧ������������£�Ϊ���CO��ת���ʿɲ�ȡ�Ĵ�ʩ���� ������
A ���� B ��ѹ C ʹ�ô��� D ���״��ӻ����ϵ�з������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�갲��ʡ���������ѧ�߶���ѧ����������3��ѧ�Ծ����������� ���ͣ������
(14��)(2011�����ݸ߶����)��25 ��ʱ����ʯī�缫���2.0 L 0.5 mol��L��1 CuSO4��Һ��5 min����һ��ʯī�缫����6.4 g Cu���ɡ��Իش��������⣺
(1)����������Ӧ����________�����缫��ӦʽΪ____________________________
________________________________________________________________________��
(2)��������Һ��������䣬�������Һ��pHΪ________��
(3)������Һ�ָ�������ǰһ�����������________mol��________��
(4)���õ�����������ͭƬ����ʯī���缫��������ͭƬ���������________g�����Һ��pH________��(���С����������䡱)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ������ʡ����У������һ��ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
(14��)ij��ȤС��ͬѧ��ʵ�����ü���1-������ŨH2SO4���廯�ƻ����ķ������Ʊ�1-�嶡�飬�����鷴Ӧ�IJ��ָ�����������ͼ��ʾװ�ã����мг�������������������ȴˮ��û�л�����
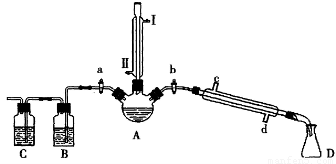
�����ʵ�鲽�裬�ش��������⣺
��1���ر�a��b����ͨ��ֱ�����ܵ�����ˮ����A����30���ӣ��Ʊ�1-�嶡�顣
��ֱ�����ܽ�ͨ����ˮ����ˮ����_____(����I����������)��������������ҪĿ����________��
��2�������ϣ�������Ӧ�ĸ���������У�����(CH3CH2CH2CH2-O-CH2CH2CH2CH3)��1-��ϩ���廯�⡢�������ơ�ˮ�ȡ�Ϩ��ƾ��ƣ�����ֱ�������Ϸ��������Ӳ���a���������ȼ�����Ӧֱ����ȴ��ͨ��B��Cװ�ü��鲿�ָ����
B��C��Ӧʢ�ŵ��Լ��ֱ���_________��????????? ��д��Cװ������Ҫ�Ļ�ѧ����ʽ��___________��
��3��Ϊ�˽�һ�������ᴿ1-�嶡�飬����ȤС��ͬѧ�������л�������������ʾ��
���� | �۵�/�� | �е�/�� |
1-���� | -89��5 | 117��3 |
1-�嶡�� | -112��4 | 101��6 |
���� | -95��3 | 142��4 |
1-��ϩ | -185��3 | -6��5 |
���㲹������ʵ�鲽�裬ֱ�������1-�嶡�顣
������ƿ��ȴ��ȥ��ֱ�������ܣ�
��____����_____����_______����_______���ռ�������֡�
��4����ʵ������ȡ1-������NaBr�ֱ�Ϊ7��4 g��13��0 g�������Ĵֲ��ᆳϴ�ӡ�������ٴ�����õ�9��6 g1-�嶡�飬��1-�嶡��IJ�����_____��������2λ��Ч���֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��ӱ�ʡ��һ��ѧ��һ�����Ի�ѧ�Ծ��������棩 ���ͣ������
����14�֣�
��1�����в����������_________������ţ�
A. ����������м�������ʵ��ʱ������ʯ������
B. Ũ�����մ��Ƥ���ϣ�Ѹ����NaOH��Һ��ϴ������ˮϴ��
C. ����ʱ�����¶ȼ�ˮ�������Һ�����¡�
D. ��������ζʱ�������������������ȶ���ʹ��������Ʈ��ǿ��С�
E. ʹ�÷�Һ©��������ƿʱ�����ȼ���Ƿ�©Һ��
(2) ���ý�屨���˶�����������ϴʱ������ʹ�á�����顱����Ҫ�ɷ������ᣩ�롰84
����Һ������Ч�ɷ���NaClO�����������ж����¼�����������ѧ��������ԭ��Ӧ֪ʶ����
���ӷ���ʽ��ʾ����������ԭ��
(3) ��֪��ƫ��������Һ�м���ϡ�����������������ɡ�ijͬѧ������ѧ֪ʶ���������ʹ�ø÷�����ȡA1(OH)3������Ϊ ���������ӷ���ʽ��ʾ���������ٳ�һ�����Ȼ�����Һ��ȡA1(OH)3�ĺ����������÷�Ӧ�����ӷ���ʽΪ ��
(4)���йر�����Ŀǰ����ұ�������ȸߴ�99��9999�����������й��ڴ����������У���ȷ���� ������ĸ����
A��Ӳ�ȱȸ�С���۵�ȸָ� B�����������ᷴӦ
C���벻��ֳɷ���ͬ D�������Ũ�����жۻ�
(5)���ˮ����εμӱ���FeCl3��Һ����Һ������ĺ��ɫʱֹͣ���ȣ����÷�ɢϵ�ķ�ɢ�ʴ�С��Χ�� ��
(6)�ϳɰ���ҵ�����г�������ý������������Ҫ�ɷ���FeO��Fe2O3����֪ij����ý�У����������ӵ����ʵ���֮��Ϊ4��5������Fe2+��Fe3+���ʵ���֮��Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com