
·“Ó¦2C£«O2===2COµÄÄÜĮæ±ä»ÆČēĶ¼ĖłŹ¾”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ(””””)
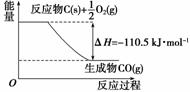
A£®12 g C(s)ÓėŅ»¶ØĮæO2(g)·“Ӧɜ³É14 g CO(g)£¬·Å³öµÄČČĮæĪŖ110.5 kJ
B£®2 mol C(s)Óė×ćĮæO2(g)·“Ӧɜ³ÉCO2(g)£¬·Å³öµÄČČĮæ“óÓŚ221 kJ
C£®øĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ŹĒ2C(s)£«O2(g)===2CO(g)
¦¤H£½£221 kJ
D£®øĆ·“Ó¦µÄ·“Ó¦ČȵČÓŚCO·Ö×ÓÖŠ»Æѧ¼üŠĪ³ÉŹ±ĖłŹĶ·ÅµÄ×ÜÄÜĮæÓėO2·Ö×ÓÖŠ»Æѧ¼ü¶ĻĮŃŹ±ĖłĪüŹÕµÄ×ÜÄÜĮæµÄ²ī
“š°ø””B
½āĪö””øł¾ŻĶ¼æÉÖŖ12 g C(s)ÓėŅ»¶ØĮæO2(g)·“Ӧɜ³É28 g CO(g)·Å³öµÄČČĮæĪŖ110.5 kJ£¬AĻī“ķĪó£»2 mol C(s)Óė×ćĮæO2(g)·“Ӧɜ³ÉCO(g)·Å³öČČĮæĪŖ221 kJ£¬ÓÉÓŚCO(g) ÓėO2(g)·“Ӧɜ³ÉCO2(g)·ÅČČ£¬Ņņ“Ė2 mol C(s)Óė×ćĮæO2(g)·“Ӧɜ³ÉCO2(g)·Å³öČČĮæ“óÓŚ221 kJ£¬BĻīÕżČ·£»øĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ŹĒ2C(s)£«O2(g)===2CO(g)””¦¤H£½£221 kJ·mol£1£¬CĻī“ķĪó£»øĆ·“Ó¦µÄ·“Ó¦ČȵČÓŚCÓėO2¶Ļ¼üĪüŹÕµÄ×ÜÄÜĮæÓėCO³É¼ü·Å³öµÄÄÜĮæµÄ²ī£¬DĻī“ķĪó”£


 ŠĀæĪ±źæģĄÖĢįÓÅŹī¼Ł×÷ŅµÉĀĪ÷ĀĆÓĪ³ö°ęÉēĻµĮŠ“š°ø
ŠĀæĪ±źæģĄÖĢįÓÅŹī¼Ł×÷ŅµÉĀĪ÷ĀĆÓĪ³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijČÜŅŗÖŠÓŠFe3+”¢Mg2+”¢Fe2+ŗĶA13+ĖÄÖÖŃōĄė×Ó£¬ČōĻņĘäÖŠ¼ÓČė¹żĮæµÄĒāŃõ»ÆÄĘČÜŅŗ£¬½Į°čŗó£¬ŌŁ¼ÓČė¹żĮæµÄŃĪĖį£¬ČÜŅŗÖŠ“óĮæ¼õÉŁµÄŃōĄė×ÓŹĒ ( )
A£®Fe3+ B£®Mg2+ C£®Fe2+ D£®A13+
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
µ±CH3COOH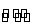 H++CH3COO-ŅŃ“ļĘ½ŗā£¬ČōŅŖŹ¹“×ĖįµÄµēĄė¶ČŗĶČÜŅŗµÄpH¶¼¼õŠ”£¬Ó¦¼ÓČėµÄŹŌ¼ĮŹĒ( )
H++CH3COO-ŅŃ“ļĘ½ŗā£¬ČōŅŖŹ¹“×ĖįµÄµēĄė¶ČŗĶČÜŅŗµÄpH¶¼¼õŠ”£¬Ó¦¼ÓČėµÄŹŌ¼ĮŹĒ( )
A.CH3COONa B.NH3”¤H2O C.HCl D.H2O
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ė®ŹĒŅ»ÖÖ¼«ČõµÄµē½āÖŹ£¬ŌŚ³£ĪĀĻĀĘ½¾łĆænøöĖ®·Ö×ÓÖ»ÓŠ1øö·Ö×Ó·¢ÉśµēĄė£¬nµÄÖµŹĒ( )
A.1”Į1014 B.55.6”Į107
C.1”Į107 D.55.6
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
1,3¶”¶žĻ©ŗĶ2¶”Č²·Ö±šÓėĒāĘų·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ČēĻĀ£ŗ
CH2===CH—CH===CH2(g)£«2H2(g)”Ŗ”śCH3CH2CH2CH3(g)£«236.6 kJ
CH3—C”ŌC—CH3(g) £« 2H2(g)”Ŗ”śCH3CH2CH2CH3(g)£«272.7 kJ
ÓÉ“Ė²»ÄÜÅŠ¶Ļ
A£®1,3¶”¶žĻ©ŗĶ2¶”Č²ĪČ¶ØŠŌµÄĻą¶Ō“óŠ”
B£®1,3¶”¶žĻ©ŗĶ2¶”Č²·Ö×Ó“¢“ęÄÜĮæµÄĻą¶ŌøßµĶ
C£®1,3¶”¶žĻ©ŗĶ2¶”Č²Ļą»„×Ŗ»ÆµÄČČŠ§Ó¦
D£®Ņ»øöĢ¼Ģ¼Čž¼üµÄ¼üÄÜÓėĮ½øöĢ¼Ģ¼Ė«¼ü¼üÄÜÖ®ŗĶµÄ“óŠ”
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
2SO2(g)£«O2(g)2SO3(g)·“Ó¦¹ż³ĢµÄÄÜĮæ±ä»ÆČēĶ¼ĖłŹ¾”£ŅŃÖŖ1 mol SO2(g)Ńõ»ÆĪŖ1 mol SO3(g)µÄ¦¤H£½£99 kJ·mol£1”£
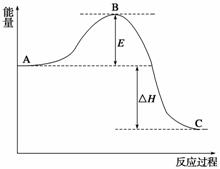
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)Ķ¼ÖŠA”¢C·Ö±š±ķŹ¾__________”¢__________£¬EµÄ“󊔶ŌøĆ·“Ó¦µÄ·“Ó¦ČČÓŠĪŽÓ°Ļģ£æ__________”£øĆ·“Ó¦Ķس£ÓĆV2O5×÷“߻ƼĮ£¬¼ÓV2O5»įŹ¹Ķ¼ÖŠBµćÉżøß»¹ŹĒ½µµĶ£æ____________£¬ĄķÓÉŹĒ_________________________________________________________£»
(2)Ķ¼ÖŠ¦¤H£½__________kJ·mol£1£»
(3)V2O5µÄ“ß»ÆŃ»·»śĄķæÉÄÜĪŖV2O5Ńõ»ÆSO2Ź±£¬×ŌÉķ±»»¹ŌĪŖĖļŪ·°»ÆŗĻĪļ£»ĖļŪ·°»ÆŗĻĪļŌŁ±»ŃõĘųŃõ»Æ”£Š“³öøĆ“ß»ÆŃ»·»śĄķµÄ»Æѧ·½³ĢŹ½£ŗ________________________________________________________________________£»
(4)ŅŃÖŖ³£ĪĀĻĀ£¬1 mol¹ĢĢåĮņČ¼ÉÕÉś³É¶žŃõ»ÆĮņĘųĢåŹ±·Å³öµÄČČĮæŹĒ296 kJ£¬¼ĘĖćÓÉS(s)Éś³É3 mol SO3(g)µÄ¦¤H________(ŅŖĒ󊓳ö¼ĘĖć¹ż³Ģ)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠČČ»Æѧ·½³ĢŹ½ŹéŠ“ÕżČ·µÄŹĒ(””””)
A£®2SO2£«O2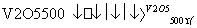 2SO3””¦¤H£½£196.6 kJ·mol£1
2SO3””¦¤H£½£196.6 kJ·mol£1
B£®H2(g)£«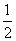 O2(g)===H2O(l)
O2(g)===H2O(l)
¦¤H£½£285.8 kJ·mol£1
C£®2H2(g)£«O2(g)===2H2O(l)””¦¤H£½£571.6 kJ
D£®C(s)£«O2(g)===CO2(g)””¦¤H£½£«393.5 kJ·mol£1
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠŠšŹöÖŠÕżČ·µÄŹĒ £Ø £©
A£®Ė®²£Į§µÄÖ÷ŅŖ³É·ÖŹĒ¹čĖį
B£®ĻąĶ¬ĪĀ¶ČĻĀ£¬Na2CO3µÄČܽā¶ČŠ”ÓŚNaHCO3
C£®H2O2ÖŠHŌŖĖŲµÄ»ÆŗĻ¼ŪŹĒ+2
D£®“×ĖįµÄµēĄė·½³ĢŹ½£ŗCH3COOH CH3COO£ + H+
CH3COO£ + H+
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijŹµŃ銔×éµÄĶ¬Ń§¶Ōµē»ÆѧŌĄķ½ųŠŠĮĖŅ»ĻµĮŠĢ½¾æ»ī¶Æ
¢ÅČēĶ¼ĪŖijŹµŃ銔×éŅĄ¾ŻŃõ»Æ»¹Ō·“Ó¦£ŗ£ØÓĆĄė×Ó·½³ĢŹ½±ķŹ¾£© Éč¼ĘµÄŌµē³Ų×°ÖĆ£¬·“Ó¦Ē°£¬Į½µē¼«ÖŹĮæĻąµČ£¬Ņ»¶ĪŹ±¼äŗó£¬Į½µē¼«ÖŹĮæĻą²ī18g£¬Ōņµ¼ĻßÖŠĶعż molµē×Ó”£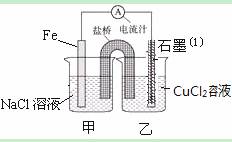
¢ĘÓĆ½ŗĶ·µĪ¹ÜĪü³öĢśĘ¬ø½½üČÜŅŗÉŁŠķÖĆÓŚŹŌ¹ÜÖŠ£¬ĻņĘäÖŠµĪ¼ÓÉŁĮæŠĀÖʱ„ŗĶĀČĖ®£¬Š“³ö·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ £¬Č»ŗóµĪ¼Ó¼øµĪĮņĒč»Æ¼ŲČÜŅŗ£¬ČÜŅŗ±äŗģ£»¼ĢŠųµĪ¼Ó¹żĮæŠĀÖʱ„ŗĶĀČĖ®£¬ŗģÉ«ĶŹČ„£¬¼ŁÉčÖ®Ņ»ŹĒ”°ČÜŅŗÖŠµÄ+3¼ŪĢś±»Ńõ»ÆĪŖøüøߵļŪĢ¬”±”£Čē¹ū+3¼ŪĢś±»Ńõ»ÆĪŖFeO42-£¬ŹŌŠ“³öøĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ ”£
¢ĒČēĶ¼ĘäĖüĢõ¼ž²»±ä£¬Čō½«ŃĪĒÅ»»³ÉĶäĶµ¼ĻßÓėŹÆÄ«ĻąĮ¬³ÉnŠĶ£¬ČēĶ¼ĖłŹ¾£¬ŹÆÄ«¢ÅĪŖ ¼«£ØĢī ”°Õż”±”¢”°øŗ”±”¢”°Ņõ”±»ņ ”°Ńō”±£©”£ŌŚ¼××°ÖĆÖŠµĪ¼Ó¼øµĪ·ÓĢŖŹŌŅŗ£¬Õńµ“¾łŌČ£¬Ņ»¶ĪŹ±¼äŗó£¬ŌŚ¼××°ÖĆÖŠ¹Ū²ģµ½ µē¼«£ØĢī”°Ģś”±»ņ”°Ķ”±£©ø½½üČÜŅŗĻȱäŗģ£¬øƵē¼«µÄ·“Ó¦Ź½ĪŖ ”£

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com