

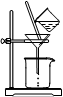

 MnCl2��2H2O��Cl2������ȥHCl������ˮ��������ʯ��
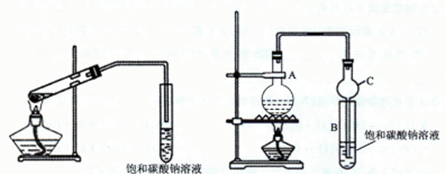
MnCl2��2H2O��Cl2������ȥHCl������ˮ��������ʯ�� MnCl2��2H2O��Cl2�����������ɵ������к��лӷ������Ȼ��⣬���Ȼ���Ũ���ź���ķ�Ӧ������װ��B�б���NaCl��Һ�����������ڳ�ȥ������HCl��������ˮAlCl3(183������)����ʪ������������������������FʢװŨ���ᣬ��ֹˮ��������E�����ڼ�ʯ�Ҽ�����ˮ��Ҳ���������������Կ��ø����ʢװ��ʯ�ҿ�����F��G�����á�
MnCl2��2H2O��Cl2�����������ɵ������к��лӷ������Ȼ��⣬���Ȼ���Ũ���ź���ķ�Ӧ������װ��B�б���NaCl��Һ�����������ڳ�ȥ������HCl��������ˮAlCl3(183������)����ʪ������������������������FʢװŨ���ᣬ��ֹˮ��������E�����ڼ�ʯ�Ҽ�����ˮ��Ҳ���������������Կ��ø����ʢװ��ʯ�ҿ�����F��G�����á�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A����ʽ�ζ���δ��ϴ | B����ʽ�ζ��ܵζ�ǰ�����ݣ��ζ���������ʧ |
| C�����������Һʱ�����ݸ��� | D����������δ��ȫˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
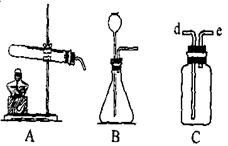
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
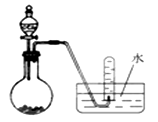
| A����Ũ������������̷�Ӧ��ȡCl2 |
| B����Ũ��ˮ����ʯ�ҷ�Ӧ��ȡNH3 |
| C���õ�ʯ��ˮ��Ӧ��ȡC2H2 |
| D����Ũ�����ͭ��Ӧ��ȡNO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 2CaSO4+2Cl2��+2H2O �����������������ȡ��������֤�����ʵ�ʵ�顣
2CaSO4+2Cl2��+2H2O �����������������ȡ��������֤�����ʵ�ʵ�顣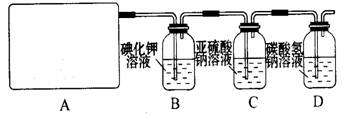
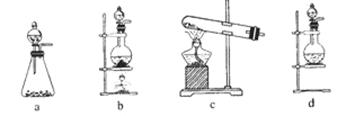
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
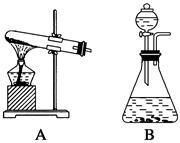
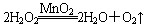 ����Ӧ��MnO2��������________����ʵ�������ô˷�Ӧ��ȡ����ʱ��Ӧѡ�õ����巢��װ����________��������鱾װ�������Եķ�����______________________________________________��
����Ӧ��MnO2��������________����ʵ�������ô˷�Ӧ��ȡ����ʱ��Ӧѡ�õ����巢��װ����________��������鱾װ�������Եķ�����______________________________________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���屽�л����壬����KI��Һ������������ȡ���� |
| B�������л�����ϩ��ͨH2��һ�������·�Ӧ��ʹ��ϩת��Ϊ���� |
| C���������л���ŨH2SO4��ŨHNO3�����䵹��NaOH��Һ�У����ã���Һ |
| D����ϩ�л���CO2��SO2������ͨ��ʢ��NaOH��Һ��ϴ��ƿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
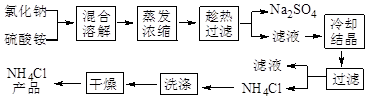
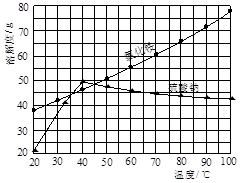
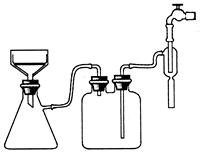
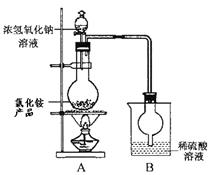
| A��������ȴ��Һ | B����ҺŨ�Ƚϸ� |
| C�������ܽ�Ƚ�С | D�����������ܼ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com