
ij����Һ�п��ܺ���NH4+��Ba2+��Na+��Fe2+��Cl����CO32����NO3�����������е����֡�ijͬѧȡ4�ݴ���Һ��Ʒ���ֱ����������ʵ�飺
����pH��ֽ�����Һ��ǿ���ԣ�
�ڼ������NaOH��Һ�������д̼�����ζ���������г������ɣ�
�ۼ��������ữ��AgNO3��Һ������ɫ������
�ܼ�����BaCl2��Һ��û�г�������������Һ�м�����ˮ���ٵμ�KSCN��Һ���Ժ�ɫ
��ͬѧ����ȷ�����������������п϶�����NH4+��Fe2+��Cl���������ӡ�
���������ͬѧֻ��Ҫ��������ļ���ʵ�飬���ɵó��˽��ۡ�
A���٢ڢ� B���٢� C���٢ڢۢ� D���ڢۢ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���� | �����Լ� | ʵ������ |
| ��һ����Һ | �μ������ĵ���KI��Һ | ����ɫ |
| �ڶ�����Һ | �μ��������ữ��BaCl2��Һ | �а�ɫ���� |
| ��������Һ | �μ�NaOH��Һ�����ȣ������NaOH��Һ�����V�������ɵij��������������壨n���Ĺ�ϵ��ͼ |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��15�֣�ij��ɫ��Һ�п��ܺ���NH4+��K+��Al3+��HCO3����Cl����MnO4����SO42���������еļ������ӡ�
�پ�ʵ���֪��Һ���Գ����ԣ�����ɫ��Ӧ���ֳ���ɫ��
��ȡ10mL����Һ���Թ��еμ�Ba(NO3)2��Һ����ϡ�����ữ����˵õ�0.03mol��ɫ����������Һ�м���AgNO3��Һδ������������
����ȡ��������Һ���Թ��У��μ�NaOH��Һ������ɫ���������������ӵ�һ������ʼ�������壬��������ȫ�ܽ⡣
��1������Һ��һ�������� �������ӵĻ�ѧʽ�����ӣ�һ�����е������� �������ӵĻ�ѧʽ����
��2������Щ�����ڵ������У���һ�����������Ի����кͼ��Ի����ж����ܴ��ڣ���д�����������ᷴӦ�����ӷ���ʽ�� ��
��3��Ϊȷ��������Һ�������ĸ������ӵ����ʵ�����ȡ100mL������Һ�������м���Na2O2���壬�����ij���������������Na2O2�������ʵ����Ĺ�ϵ������ͼ��
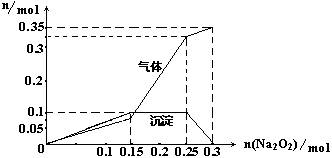 |
�ٸ���Һ����ɫ��Ӧ������ɫ�����ӵ����ʵ���Ϊ�� mol��
��д��n(Na2O2)=0.2molʱ��Ӧ�������ӷ���ʽ�� ��
��4����0.1mol��������������ʵ�����ij��ˮ�ξ���X��Ϻ��ܽ���ˮ�У�������Һ��������Һ��������������ȫ��ͬ���������Һ�м���Ba(OH)2��Һ�����ó��������ʵ�����������Ba(OH)2�����ʵ����Ĺ�ϵ��ͼ��
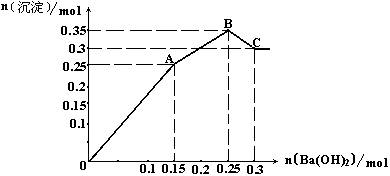 |
�Ը���ͼ���ƶϢ�X�Ļ�ѧʽ�� ����ͼ����A����Һ�е����ӳɷֺ����ʵ����ֱ��ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ����ר���̵�10�� �ǽ������仯������ϰ���������棩 ���ͣ������
�������������������γ��������Ҫ���ʡ�ij�������п��ܺ����������ӣ�Na����Ba2����NH4+��Al3����Cl����SO32-��SO42-��NO3-�ȡ�ij�о�С��ȡ�õ�һ���������꣬Ũ�������ó�����Һ�ֳ����ݣ���������ʵ�飺
���� | �����Լ� | ʵ������ |
��һ����Һ | �μ������ĵ���?KI��Һ | ��Һ����ɫ |
�ڶ�����Һ | �μ��������ữ��BaCl2��Һ | �а�ɫ�������� |
��������Һ | �μ�NaOH��Һ�����ȣ������NaOH��Һ���(V)�����ɵij�������������������ʵ���(n)�Ĺ�ϵ����ͼ |
|
��ش��������⣺
(1)����ʵ�����жϸ������п϶������ڵ�������______________������ȷ����������________________��
(2)д����һ����Һ�μӵ���?KI��Һʱ������Ӧ�����ӷ���ʽ��__________________��
(3)��������Һ�μ�NaOH��Һ�����ȣ����������з����˶����Ӧ��д������������Ӧ�����ӷ���ʽ��__________________________________��__________________________��
(4)���ʵ�鷽����������������Ƿ����Cl����___________________________________
______________________________��
(5)��С��Ϊ��̽��NO����������������γɹ��̣�����ƿ�г��뺬������NO��SO2���壬������ͨ��O2��������ѧ��Ӧ����������������ˮ�������������꣬��NO��������Ӧ�е�������________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com