
| A����Ũ�ȵ�HCN��ҺNaCN��Һ�������ϣ�������ҺpH��7������Һ������Ũ�ȣ�c��Na+ ����c��CN-����c��OH- ����c��H+ �� | ||||
| B��0.4mol?L-1ijһԪ��HA��Һ��0.2 mol?L-1NaOH��Һ�������ϵ���Һ�У�2c��OH- ��+c��A- ��=2c��H+ ��+c��HA�� | ||||
| C�������µ�Ũ�ȵ�Na2SO3��NaHSO3��Һ�������Ϻ���Һ��pH=7.2��������������Һ������ʱ��c��Na+ ����c��HSO3-����c��SO32-����c��H+ ���Tc��OH- �� | ||||
D����������HX��HY��Ϻ���Һ�е�c��H+ ��Ϊ��KaΪ����ƽ�ⳣ������c��H+ ��=
|
| Ka(HY)��c(HY) |
| c(H+) |
| Ka(HX)��c(HX) |
| c(H+) |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| n |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ʪ���pH��ֽ��ϡ��Һ��pH���ⶨֵƫС |
| B������к͵ζ�ʱ�ô���Һ��ϴ��ƿ��������ƫ�� |
| C���ζ�ǰ�ζ����������ݣ��յ����ʱ�����ݣ��������ƫС |
| D���ⶨ�кͷ�Ӧ�ķ�Ӧ��ʱ��������������У���������¶�ֵƫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢ڢۢ� | B���ڢۢ� |
| C���٢ڢ� | D���ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
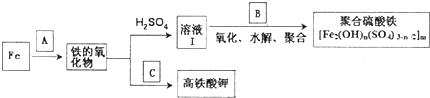
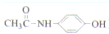 �������¼��X������õķ�������������ʹҩ���������Ƹ�ð���պؽ�ʹ����ʹ��ƫͷʹ�Ȳ�֢�����������·������ϳ�
�������¼��X������õķ�������������ʹҩ���������Ƹ�ð���պؽ�ʹ����ʹ��ƫͷʹ�Ȳ�֢�����������·������ϳ�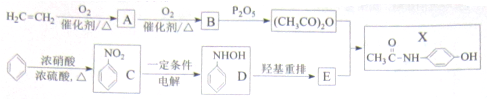
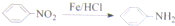 ����ش��������⣺
����ش��������⣺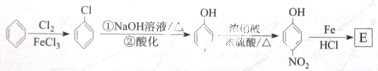
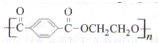 ������ƺϳ�·�ߣ����Լ����ܼ���ѡ��
������ƺϳ�·�ߣ����Լ����ܼ���ѡ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��NaHSO3��NaHCO3�����Ի����Һ�У�c��Na+��=c��HSO3-��+2c��SO32-��+c��HCO3-��+2c��CO32-�� |
| B�������½������ơ���������Һ��Ϻ���Һ�����ԣ����Ϻ���Һ�У�c��Na+����c��Cl-����c��CH3COOH�� |
| C�����������ʵ���Ũ����ȵĢ٣�NH4��2CO3���ڣ�NH4��2SO4���ۣ�NH4��2Fe��SO4��2������Һ��c��NH4+�����٣��ڣ��� |
| D����������ʵ���Ũ�ȵ�NaClO��aq����NaCl��aq���������������٣�Nǰ��N�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��4����ҺpH�Ĵ�С˳�٣��ܣ��ۣ��� |
| B����Һ�١��ڵ������Ϻ�pH��7����c��NH4+����c��NH3?H2O�� |
| C������Һ�١����зֱ����25��mL��0.1mol/L���������Һ��c��NH4+�����٣��� |
| D������Һ�ۡ����зֱ����25mL��0.1mol/L��NaOH��Һ������Һ�е�����������ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
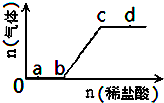 ��Na2CO3��NaHCO3�����Һ����μ���ϡ���ᣬ�����������������������ı仯��ϵ��ͼ��ʾ���������������ڶ�Ӧ����Һ��һ���ܴ���������ǣ�������
��Na2CO3��NaHCO3�����Һ����μ���ϡ���ᣬ�����������������������ı仯��ϵ��ͼ��ʾ���������������ڶ�Ӧ����Һ��һ���ܴ���������ǣ�������| A��a���Ӧ����Һ�У�Na+��SO42-��NO3-��Fe��OH��3������ |
| B��b���Ӧ����Һ�У�Al3+��H+��MnO4-��Cl- |
| C��c���Ӧ����Һ�У�Na+��Ca2+��NO3-��Cl- |
| D��d���Ӧ����Һ�У�F-��NO3-��Fe2+��Ag+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
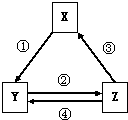 �±���������֮��ͨ��һ����Ӧ������ʵ����ͼ��ʾת����ϵ���ǣ�������
�±���������֮��ͨ��һ����Ӧ������ʵ����ͼ��ʾת����ϵ���ǣ�������| ѡ�� | X | Y | Z | ��ͷ���������ֵķ�Ӧ���� |
| A | Na2O2 | NaOH | NaCl | �ٳ�����ˮ |
| B | Al2O3 | NaAlO2 | Al��OH��3 | ��ͨ��CO2 |
| C | NO | NO2 | HNO3 | �ܼ���ͭ�� |
| D | Cl2 | NaClO | HClO | �ۼ�Ũ���� |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com