���𰸡�
��������A��B�Ļ����0.05molȼ������0.1 molCO
2��0.1molH
2O�Լ�����������֪A��B�Ļ����ƽ����ɷ���ʽΪC
2H
4O������AΪ����BΪ���ĺ����������A��BΪ�����ʵ���������ƽ��ֵ���壨��A��B��̼ԭ����ȷ���������ֿ��ܣ�һ�����������̼ԭ������Ϊ2����һ�����һ��̼ԭ��������2��һ��̼ԭ����С��2����A��B����Ͽ��������֣��ֱ���CH
4��C
3H
4O
2��C
2H
4��C
2H
4O
2��C
2H
2��C
2H
6O
2��C
2H
6��C
2H
2O
2��C
3H
6��CH
2O
2���Դ˽��з�����
����⣺��A��B�Ļ����0.05molȼ������0.1 molCO
2��0.1molH
2O�Լ�����������֪A��B�Ļ����ƽ����ɷ���ʽΪC
2H
4O������AΪ����BΪ���ĺ����������A��BΪ�����ʵ���������ƽ��ֵ���壨��A��B��̼ԭ����ȷ���������ֿ��ܣ�һ�����������̼ԭ������Ϊ2����һ�����һ��̼ԭ��������2��һ��̼ԭ����С��2����A��B����Ͽ��������֣��ֱ���CH
4��C
3H
4O
2��C
2H
4��C
2H
4O
2��C
2H
2��C
2H
6O
2��C
2H
6��C
2H
2O
2��C
3H
6��CH
2O
2��
��1��������ȼ�շ�Ӧ���ݿ�֪��1 mol�����+2.5 molO
2��2 molCO
2+2mol H
2O���������غ�ĽǶȵĿ�֪������ƽ������ʽΪC
2H
4O���ʴ�Ϊ��C
2H
4O��
��2��������AΪ����BΪ���ĺ����������B�бغ�2��Oԭ�ӣ��������������ʵ����Ȼ�ϣ������ʵ���֮��һ��ʱ������̼ԭ������Ϊ2��ӦΪCH��CH��
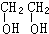
��
�ʴ�Ϊ��CH��CH��

��
�������ɵ�CO
2 �� H
2O �����ʵ���һ���������������к��е�C��Hԭ������ͬ��ӦΪCH
2=CH
2��CH
3COOH��
�ʴ�Ϊ��CH
2=CH
2��CH
3COOH��
���������⿼���л������ʽ��ȷ������Ŀ�Ѷ��еȣ������ƽ������ʽ���������ɲ���ͺ��������㣬����Ϊ������Ŀ�ĵ��ͣ�

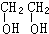 ��
�� ��
��







