

| �ζ� ���� | ����NaOH��Һ�����/mL | 0.100 0mol/L��������/mL | ||
| �ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
| ��һ�� | 25.00 | 0.00 | 25.11 | 25.11 |
| �ڶ��� | 25.00 | 0.56 | 30.56 | 30.00 |
| ������ | 25.00 | 0.22 | 25.11 | 24.89 |
���� ��1����������������״�������ص��ж������ƣ�
�ڷ��Ǵ��л���������������ʹ��ǰһ����Ҫ����Ƿ�©ˮ��
��2���ζ��������۾�Ӧע����ƿ��Һ��ɫ�ı仯��������3.1-4.4Ϊ��ɫ���ڼ�����Һ���Ի�ɫ���ݴ��жϵζ��յ���ɫ�仯��
��3�����ݣ����⣩=$\frac{V������C������}{V�����⣩}$��������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��4 �����ݵζ��ܵĽṹ�;�ȷ���Լ�������ԭ����
��5���ȸ������ݵ���Ч�ԣ���ȥ��2�����ݣ�Ȼ�����1��3��ƽ������V�����ᣩ�����Ÿ��������NaOH��Ӧ���C��NaOH����
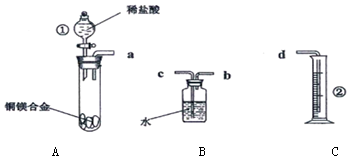
��� �⣺��1��������B����ƿ������ƿ��ϸ���п̶����̶�Ϊ����ƿ��C���п̶ȣ�С�����ϣ��������£�Ϊ�ζ��ܣ�
�ʴ�Ϊ������ƿ���ζ��ܣ�
������ƿ���л������ζ��ܴ���������Ϊ��ֹ©Һ��ʹ��ǰӦ����Ƿ�©ˮ��
��ѡ��BC��
��2���ζ��������۾�Ӧע����ƿ��Һ��ɫ�ı仯���ñ�������ζ������NaOH��Һʱ���ü�����ָʾ������ʼ��Һ�Լ��ԣ�������ƿ����Һ��ɫΪ��ɫ����������ļ��������࣬��Һ���Լ�������ɫ��dz�����ɻ�ɫͻ��Ϊ��ɫ���Ұ��������ɫ����ԭ�����ﵽ�յ㣻
�ʴ�Ϊ����ƿ����Һ��ɫ�仯����ɫͻ��Ϊ��ɫ��
��3��A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע������ᣬ���±�Һ��ϡ�ͣ������Һ���ƫ�����ݣ����⣩=$\frac{V������C������}{V�����⣩}$��֪ ��ҺŨ��ƫ�ߣ���A��ѡ��
B���ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и�������ı�Һ�����������Ӱ�죬���ݣ����⣩=$\frac{V������C������}{V�����⣩}$��֪��ҺŨ����Ӱ�죬��B��ѡ��
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ���������ĵı�Һ���ƫ�����ݣ����⣩=$\frac{V������C������}{V�����⣩}$��֪��ҺŨ��ƫ�ߣ���C��ѡ��
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ��������V������ƫС�����ݣ����⣩=$\frac{V������C������}{V�����⣩}$��֪��ҺŨ��ƫ�ͣ���Dѡ��
��ѡ��D��
�� 4���ζ���С�̶����ϣ���̶����£���ȷֵΪ0.01mL����ʼ����Ϊ0.00mL���յ����Ϊ26.10mL��������Һ�����Ϊ26.10mL��
�ʴ�Ϊ��26.10��
��5���������ݵ���Ч�ԣ���ȥ��2�����ݣ�Ȼ�����1��3��ƽ������V�����ᣩ=25.00mL��
�����ݣ����⣩=$\frac{V������C������}{V�����⣩}$=$\frac{0.1000mol/L��25.00mL}{25.00mL}$=0.10mol/L��
�ʴ�Ϊ��0.10mol/L��
���� ������Ҫ�������к͵ζ��������������Լ����㣬��ȷ�к͵ζ���ԭ�������������ǽ���ؼ���ע���������ķ�������Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| PCl3��g�� | Cl2��g�� | PCl5��g�� | |
| ��ʼŨ��/��mol/L�� | 2.0 | 1.0 | 0 |
| ƽ��Ũ��/��mol/L�� | c1 | c2 | 0.4 |
| A�� | 10 min�ڣ�v��Cl2��=0.04 mol/��L•min�� | |
| B�� | �����¶ȣ���Ӧ��ƽ�ⳣ����С����ƽ��ʱPCl3��ת���ʱ�� | |
| C�� | ��������Cl2Ϊ1.2 molʱ����Ӧ�ﵽƽ�� | |
| D�� | ƽ�������2.0 mol PCl3��1.0 mol Cl2������ͬ�������ٴ�ƽ��ʱ��c��PCl5����0.2 mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| IA | 0 | ||||||||
| 1 | IIA | IIIA | IVA | VA | VIA | VIIA | |||
| 2 | �� | �� | Ne | ||||||
| 3 | �� | �� | �� | �� |
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �¶ȣ��棩 | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
| ͭƽ���ܽ����� ��10-3mol•L-1•min-1�� | 7.34 | 8.01 | 9.25 | 7.98 | 7.24 | 6.73 | 5.76 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | v��A��=0.7 mol/��L��min�� | B�� | v��B��=0.3mol/��L��min�� | ||
| C�� | v��C��=0.9 mol/��L��min�� | D�� | v��D��=1.1mol/��L��min�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
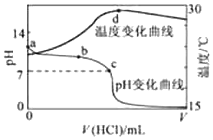 �����£���1.000mol•L-1�������20.00mL 1.000mol•L-1��ˮ�У���ҺpH���¶��������������仯������ͼ��ʾ�������й�˵����ȷ���ǣ�������
�����£���1.000mol•L-1�������20.00mL 1.000mol•L-1��ˮ�У���ҺpH���¶��������������仯������ͼ��ʾ�������й�˵����ȷ���ǣ�������| A�� | a����ˮ�������c��H+��=1.0��10-14mol/L | |
| B�� | b�㣺c��NH4+��+c��NH3•H2O����c��Cl-�� | |
| C�� | b��ʱ������������С��20.00mL����c��ʱ����������������20.00mL | |
| D�� | d�����Һ�¶����½�����Ҫԭ����NH3•H2O�������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com