
��ͼ��һ���Ҵ�ȼ�ϵ�ع���ʱ��ʾ��ͼ���ҳ��е������缫һ����ʯī�缫��һ�������缫������ʱM��N�����缫�������������٣���ش��������⣺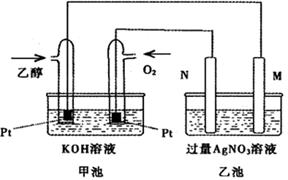
��1��M�缫�IJ����� ���缫������ �������Ҵ��IJ��缫�ĵ缫��Ӧ
ʽΪ ��д���ҳ��з����Ļ�ѧ��Ӧ�����ӷ���ʽ�� ��
��2���ڴ˹����У��ҳ���ijһ�缫����������4��32gʱ���׳�����������������Ϊ L����״���£�������ʱ�ҳ���Һ�����Ϊ400mL�����ҳ�����Һ��pHΪ ��
��3�����ڳ��³�ѹ�£�1g C2H5OHȼ������CO2��Һ̬H2Oʱ�ų�29��71kJ��������ʾ�÷�Ӧ���Ȼ�ѧ����ʽΪ ��
��4������Ҳ��һ�ֺܺõ������Դ���̲��ں��ġ���ȼ�����Ǹ�ѹ���γɵ��������ļ���ˮ������塣��������ȼ�յ��Ȼ�ѧ����ʽΪ��CH4(g)��2O2(g)��CO2(g)��2H2O(l) ��H����890��3 kJ��mol��356g����ȼ����(������ʽΪCH4��9H2O)�ͷŵļ���������ȫȼ������Һ̬ˮ���ų�������Ϊ kJ��
��1���� ���� C2H5OH��12e+16OH��=2CO32��+11H2O 4Ag++2H2O 4Ag+4H++O2����
4Ag+4H++O2����
��2��0��224 1 ��3��C2H5OH��l��+3O2��g�� 2CO2��g��+3H2O��l����H=��1366��7kJ/mol ��4��1780��6
���������������1����ͼ�ɿ���������ȼ�ϵ�ء�����ԭ��ء������ǵ��ء����ڼ׳���˵��ͨ���Ҵ��ĵ缫ʽ������ͨ�������ĵ缫�������������ҳ���˵��M��������N���������ҳ��е������缫һ����ʯī�缫��һ�������缫������ʱM��N�����缫�������������٣�˵�����缫��������M����ʯī�缫��������N��������Ϊ������缫���������ͻᷢ��������Ӧ�����������١������Ҵ��IJ��缫�ĵ缫��Ӧ��C2H5OH��12e+16OH��=2CO32��+11H2O���ҳؾ��ǵ����������Һ�������Ļ�ѧ��Ӧ�����ӷ���ʽ��4Ag++2H2O 4Ag+4H++O2������2���������պϻ�·�е���ת����Ŀ��ȡ�n(Ag)=m/M=4��32g��108g/mol=0��04mol��n(e-)=0��04mol������n(O2)=0��04mol��4=0��01mol��y��Ϊn=V/VM,����V=n��VM=0��01mol��22��4mol/L=0��224L����Ϊn(Ag)=n(H+)=0��04mol��V(aq)=0��4L����C(H+)=n/V��aq��=0��04mol��0��4L=0��1mol/L����PH=1��3���Ҵ�����Է���������46�����ڳ��³�ѹ�£�1g C2H5OHȼ������CO2��Һ̬H2Oʱ�ų�29��71kJ������46g�Ҵ�1molȼ������CO2��Һ̬H2Oʱ�ų�����46��29��71kJ=1366��7kJ����ʾ�÷�Ӧ���Ȼ�ѧ����ʽΪ��C2H5OH��l��+3O2��g��
4Ag+4H++O2������2���������պϻ�·�е���ת����Ŀ��ȡ�n(Ag)=m/M=4��32g��108g/mol=0��04mol��n(e-)=0��04mol������n(O2)=0��04mol��4=0��01mol��y��Ϊn=V/VM,����V=n��VM=0��01mol��22��4mol/L=0��224L����Ϊn(Ag)=n(H+)=0��04mol��V(aq)=0��4L����C(H+)=n/V��aq��=0��04mol��0��4L=0��1mol/L����PH=1��3���Ҵ�����Է���������46�����ڳ��³�ѹ�£�1g C2H5OHȼ������CO2��Һ̬H2Oʱ�ų�29��71kJ������46g�Ҵ�1molȼ������CO2��Һ̬H2Oʱ�ų�����46��29��71kJ=1366��7kJ����ʾ�÷�Ӧ���Ȼ�ѧ����ʽΪ��C2H5OH��l��+3O2��g�� 2CO2��g��+3H2O��l����H=��1366��7kJ/mol ��4����ȼ����Է�������Ϊ178��n(��ȼ��)=356g��108g/mol=2mol������ȫȼ������Һ̬ˮ���ų�������Ϊ2mol��890��3 kJ��mol=1780��6KJ
����ԭ��ء����ؼ��Ȼ�ѧ����ʽ����д��֪ʶ��


 ������ѧ��ʱ��ҵϵ�д�
������ѧ��ʱ��ҵϵ�д� ���������ʱ��ѵϵ�д�
���������ʱ��ѵϵ�д� �㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
�㽭�¿γ���άĿ�������ʱ��ѵϵ�д� ��������ϵ�д�
��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������һ�����͵���ɫ��Դ������һ����Ҫ�Ļ���ԭ�ϡ�
��1������ȼ����ֵ�ߡ�ʵ���ã��ڳ��³�ѹ�£�1 g H2��ȫȼ������Һ̬ˮ���ų�142.9 kJ��������H2ȼ���ȵĻ�ѧ����ʽΪ ��
��2�������Ǻϳɰ�����Ҫԭ�ϣ��ϳɰ���Ӧ���Ȼ�����ʽ���£�N2(g)��3H2(g) 2NH3(g)����H����92.4 kJ/mol
2NH3(g)����H����92.4 kJ/mol
�ٵ��ϳɰ���Ӧ�ﵽƽ��ı�ijһ�������(���ı�N2��H2��NH3����)����Ӧ������ʱ��Ĺ�ϵ����ͼ��ʾ��ͼ��t1ʱ����ƽ���ƶ������������� ���б�ʾƽ��������NH3�ĺ�����ߵ�һ��ʱ���� 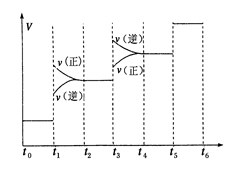
���¶�ΪT ��ʱ����2 a mol H2��a mol N2����0.5 L�ܱ������У���ַ�Ӧ����N2��ת����Ϊ50%����Ӧ��ƽ�ⳣ��Ϊ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ա���Ϊȼ����������ȼ�ϵ�أ���ص�����ͨ��O2��CO2������ͨ����飬�����������̼���Σ�����ܷ�Ӧ����ʽΪ��C3H8 +5O2 = 3CO2+ 4H2O��
��1����֪�� 2C3H8(g) + 7O2(g) =" 6CO(g)" + 8H2O(l) ?H1
C(s) + O2(g) = CO2 (g) ?H2
2C(s) + O2(g) = 2CO(g) ?H3
��C3H8(g) +5O2((g) = 3CO2(g) + 4H2O(l) ?H= ����?H1��?H2��?H3��ʾ��
��2��д���õ�������ĵ缫��Ӧʽ�� ����ع���ʱCO32������ ���øõ�ص��1000 mL 1mol/L��AgNO3��Һ���˵��صķ�Ӧ����ʽΪ �����������0��005 mol C3H8ʱ,�������Һ��pHΪ ����Һ����仯���Բ��ƣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪������Һ��CrO42���Ի�ɫ��Cr2O72-�ԳȺ�ɫ
��PbCrO4������ˮ��Ҳ������ǿ��
��H+(aq��+OH-��aq��=H2O(l���� ��H=" ��a" KJ/mol
3Cl2��g��+2Cr3+��aq��+16OH-(aq��=2CrO42-��aq��+6Cl-��aq��+8H2O(l)����H="��b" KJ/mol
2CrO42-��aq��+2H+(aq��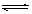 Cr2O72-��aq��+H2O(l������H="��c" KJ/mol
Cr2O72-��aq��+H2O(l������H="��c" KJ/mol
ƽ�ⳣ��K=9��5��104 ������a��b��c������0��
��������Ӧ�ݣ�ȡ50mL��Һ�������飬���ֲⶨ�������£�
| ʱ�䣨s�� | 0 | 0��01 | 0��02 | 0��03 | 0��04 |
| n ��CrO42������mol�� | 0��01 | 8��0��10-4 | 5��4��10-4 | 5��0��10-4 | |
| n ��Cr2O72������mol�� | 0 | | 4��73��10-3 | | 4��75��10-3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������Ȼ�ѧ����ʽ����ѧ����ʽ���缫��Ӧʽ������ʽ�ȣ�����д��
��1����֪��2Cu(s)��1/2O2(g)=Cu2O(s)����H=-169kJ��mol-1��
C(s)��1/2O2(g)=CO(g)����H=-110.5kJ��mol-1��
Cu(s)��1/2O2(g)=CuO(s)����H=-157kJ��mol-1
��̿���ڸ��������»�ԭCuO����Cu2O���Ȼ�ѧ����ʽ�ǣ�
��2����һ�������£���������������������·�Ӧ��2SO2(g)+O2(g) 2SO3(g)��д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ��
2SO3(g)��д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ��
��3���Լ��顢����Ϊ��Ӧ�KOH��Һ���������Һ����ȼ�ϵ�أ�����ӦʽΪ�� ��
��4�����ڳ�ʪ�Ŀ����з���������ʴ�ĵ�ط�Ӧ����ʽΪ ��
��5����þ���������Ρ�ȼ�ϵ�أ���װ��ʾ��ͼ��ͼ���õ�ط�Ӧ���ܷ�Ӧ����ʽΪ_______________��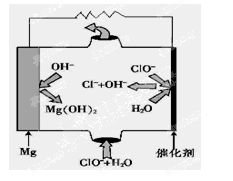
��6����ҵ�ϵ�������Ȼ��Ƶķ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����298Kʱ��1molC2H6����������ȫȼ�����ɶ�����̼��Һ̬ˮ�ų�����1558��3 kJ��д���÷�Ӧ���Ȼ�ѧ����ʽ ��
��2�����ø÷�Ӧ���һ��ȼ�ϵ�أ�������������Һ���������Һ�����ʯī���缫���ڵ缫�Ϸֱ�
ͨ�������������ͨ����������ĵ缫ӦΪ ������д���������������õ缫�Ϸ����ĵ缫��Ӧ
ʽ��
��3����ͼ��ʾʵ��װ���У�ʯī���ϵĵ缫��ӦʽΪ �������ʼʱʢ��1000mLpH=5������ͭ��Һ��25�棬CuSO4��������һ��ʱ�����Һ��pH��Ϊ1����ʱ��Ҫʹ��Һ�ָ�����ʼŨ�ȣ��¶Ȳ��䣬������Һ����ı仯����������Һ�м��� __________�����������ƣ���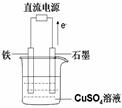
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ����CO2��H2��һ�������������·�Ӧ�ϳɼ״����ų��������ȣ�CO2(g)+3H2(g) CH3OH(g)+H2O(g) ��H1 �ش��������⡣
CH3OH(g)+H2O(g) ��H1 �ش��������⡣
��1����֪��2H2(g)+O2(g)=2H2O(g) ��H2
��Ӧ2CH3OH(g)+3O2(g)=2CO2(g)+4H2O(g) ��H= ���ú���H1����H2��ʾ��
��2������Ӧ�¶����ߣ�CO2��ת���� (�������С�����䡱����
��3��д�������Ի����У��״�ȼ�ϵ���е�������Ӧ����ʽ
�������״���ԭ��H2�������·����Ƶã�CH4(g) + H2O(g)  CO(g) + 3H2(g)��һ���¶��£���2 mol CH4��4 mol H2Oͨ���ݻ�Ϊ10L���ܱշ�Ӧ���У���Ӧ��CO�����ʵ���Ũ�ȵı仯�����ͼ��ʾ����ش��������⣺
CO(g) + 3H2(g)��һ���¶��£���2 mol CH4��4 mol H2Oͨ���ݻ�Ϊ10L���ܱշ�Ӧ���У���Ӧ��CO�����ʵ���Ũ�ȵı仯�����ͼ��ʾ����ش��������⣺
��4����Ӧ���е�4���ӵ���ƽ�⡣�����ӷ�Ӧ��ʼ���ո�ƽ�⣬ƽ����Ӧ����v(H2)Ϊ ������˷�Ӧ�ڴ��¶��µ�ƽ�ⳣ�����ڴ����Ӧ�ķ�����д��������̣���
��5���ڵ�5����ʱ�����������˲����Сһ������ڵ�8����ʱ�ﵽ�µ�ƽ�⣨��ʱCO��Ũ��ԼΪ0.25 mol��L��1 ��������ͼ�л�����5���Ӻ�H2Ũ�ȵı仯���ߡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���£��ݻ�Ϊ1 L���������£�����Է�������ת�����䷴Ӧ���̺�������ϵ��ͼ1��ʾ(��֪��2SO2(g)��O2(g)  2SO3(g) ��H����196.6 kJ��mol��1)����ش��������⣺
2SO3(g) ��H����196.6 kJ��mol��1)����ش��������⣺
(1)д���ܱ�ʾ���ȼ���ȵ��Ȼ�ѧ����ʽ��______________________��
(2)��H2��__________kJ��mol��1��
��.��ҵ�ϳ����ô�����Ҵ��ϳ��л��ܼ�����������
CH3COOH(l)��C2H5OH(l)  CH3COOC2H5(l)��H2O(l) ��H����8.62 kJ��mol��1
CH3COOC2H5(l)��H2O(l) ��H����8.62 kJ��mol��1
��֪CH3COOH��C2H5OH��CH3COOC2H5�ķе�����Ϊ118 �桢78 ���77 �档������������ͬʱ��ij�о�С������˶��ʵ�飬ʵ������ͼ��ʾ��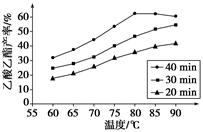
(1)���о�С���ʵ��Ŀ����___________________________________��
(2)60 ���·�Ӧ40 min��70 ���·�Ӧ20 min��ȣ�ǰ�ߵ�ƽ����Ӧ����________����(�С�ڡ��������ڡ����ڡ�)��
(3)��ͼ��ʾ����Ӧʱ��Ϊ40 min���¶ȳ���80 ��ʱ���������������½���ԭ�������_________________________________(д������)��
��.ú�����г����о���ͬ�¶���ƽ�ⳣ����Ͷ�ϱȼ���ֵ�����⡣
��֪��CO(g)��H2O(g)  H2(g)��CO2(g)ƽ�ⳣ�����¶ȵı仯���±���
H2(g)��CO2(g)ƽ�ⳣ�����¶ȵı仯���±���
| �¶�/�� | 400 | 500 | 800 |
| ƽ�ⳣ��K | 9.94 | 9 | 1 |
| | n(CO) | n(H2O) | n(H2) | n(CO2) |
| A | 1 | 5 | 2 | 3 |
| B | 2 | 2 | 1 | 1 |
| C | 3 | 3 | 0 | 0 |
| D | 0.5 | 2 | 1 | 1 |
| E | 3 | 1 | 2 | 1 |
 2CO(g)ƽ�ⳣ��ΪK��
2CO(g)ƽ�ⳣ��ΪK�� CO(g)��H2(g) ƽ�ⳣ��ΪK1��
CO(g)��H2(g) ƽ�ⳣ��ΪK1�� H2(g)��CO2(g) ƽ�ⳣ��ΪK2��
H2(g)��CO2(g) ƽ�ⳣ��ΪK2���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���Ṥҵ�Ļ����ǰ��Ĵ��������ڴ��������·������·�Ӧ��
�� 4NH3(g)+5O2(g) 4NO(g)+6H2O(g) ��H =" ��905" kJ/mol ������Ӧ
4NO(g)+6H2O(g) ��H =" ��905" kJ/mol ������Ӧ
�� 4NH3(g)+3O2(g) 2N2(g)+6H2O(g) ��H =" ��1268" kJ/mol �ڸ���Ӧ
2N2(g)+6H2O(g) ��H =" ��1268" kJ/mol �ڸ���Ӧ
�й����ʲ������¶ȵĹ�ϵ���ͼ��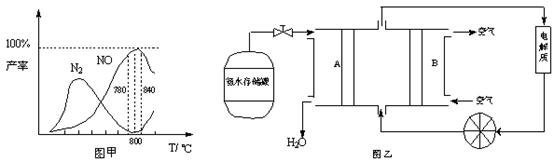
��1���ɷ�Ӧ�٢ڿ�֪��Ӧ��N2(g) + O2(g) 2NO(g)�ķ�Ӧ�Ȧ�H=
2NO(g)�ķ�Ӧ�Ȧ�H=
��2����ͼ��֪��ҵ�ϰ����������� NOʱ����Ӧ�¶���ÿ�����
��3����Fe3O4�Ʊ�Fe��NO3��3��Һʱ����ӹ�����ϡ���ᣬԭ��һ����Fe4O3�е�Fe2+ȫ��ת��ΪFe3+��
ԭ����� �������ֺ����ӷ���ʽ˵������
��4����NH3ͨ��NaClO��Һ�У�������N2H4����Ӧ�����ӷ���ʽΪ ��
��5�����ݷ�Ӧ�ڿ�����Ƴ�ֱ�ӹ���ʽ����ȼ�ϵ�أ�����ͼ��ʾ������ͼ��AΪ ��������������������缫����ʽΪ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com