

| �������� | Fe3+ | Fe2+ | Cu2+ |
| �������↑ʼ����ʱ��pH | 1.9 | 7.0 | 4.7 |
| ����������ȫ����ʱ��pH | 3.2 | 9.0 | 6.7 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ӦaA��g��+bB��g��?cC��g������H��0���ڵ��������½��У��ı�������Ӧ��������I�������ϵ�и�����Ũ����ʱ��仯��������ͼ��ʾ������˵������ȷ���ǣ�������
��ӦaA��g��+bB��g��?cC��g������H��0���ڵ��������½��У��ı�������Ӧ��������I�������ϵ�и�����Ũ����ʱ��仯��������ͼ��ʾ������˵������ȷ���ǣ�������| A����Ӧ�Ļ�ѧ����ʽ�У�a��b��c=1��3��2 |
| B��A��ƽ����Ӧ����v����A����v����A����v����A����������v����A�� |
| C���ڢ�η�Ӧ�¶�С�ڵڢ�η�Ӧ�¶� |
| D���ɵ�һ��ƽ��ڶ���ƽ�⣬��ȡ�Ĵ�ʩ�Ǵӷ�Ӧ��ϵ������C |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
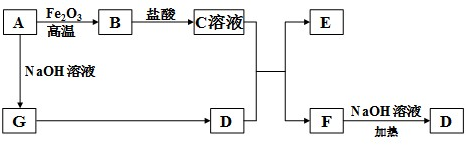
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 20.00mLһ�����ʵ���Ũ�ȵ�����X����һ��Ũ�ȵ�NaOH��ҺY�ζ����ζ���������ҺpH������NaOH��Һ������Ĺ�ϵ��ͼ��ʾ��������仯���Բ��ƣ�
20.00mLһ�����ʵ���Ũ�ȵ�����X����һ��Ũ�ȵ�NaOH��ҺY�ζ����ζ���������ҺpH������NaOH��Һ������Ĺ�ϵ��ͼ��ʾ��������仯���Բ��ƣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ���÷�Ӧ��Cu2Cl2+C2H2+2NH3��Cu2C2����Ȳ��ͭ����ɫ��+2NH4Cl�ɼ�����Ȳ��
���÷�Ӧ��Cu2Cl2+C2H2+2NH3��Cu2C2����Ȳ��ͭ����ɫ��+2NH4Cl�ɼ�����Ȳ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��NaA��NaB��NaC |
| B��NaB��NaA��NaC |
| C��NaB��NaC��NaA |
| D��NaC��NaA��NaB |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com