
ŗ¬±½·ÓµÄ¹¤Ņµ·ĻĖ®“¦ĄķµÄĮ÷³ĢĶ¼ČēĻĀ£ØŅŃÖŖĖįŠŌ£ŗH2CO3> 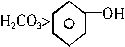 >NaHCO3£©£ŗ
>NaHCO3£©£ŗ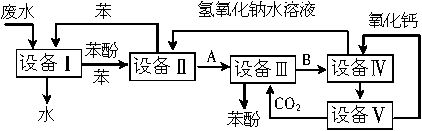
£Ø1£©ÉĻŹöĮ÷³ĢĄļ£¬Éč±ø¢ńÖŠ½ųŠŠµÄŹĒ_________²Ł×÷£ØĢīŠ“²Ł×÷Ćū³Ę£©”£ŹµŃéŹŅĄļÕāŅ»²½²Ł×÷æÉŅŌÓĆ_________½ųŠŠ£ØĢīŠ“ŅĒĘ÷Ćū³Ę£©”£
£Ø2£©ÓÉÉč±ø¢ņ½ųČėÉč±ø¢óµÄĪļÖŹAŹĒ_________”¢ÓÉÉč±ø¢ó½ųČėÉč±ø¢ōµÄĪļÖŹBŹĒ_________”£
£Ø3£©ŌŚÉč±ø¢óÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ____________”£
£Ø4£©Éč±ø¢ōÖŠ£¬BµÄĖ®ČÜŅŗŗĶCaO·“Ó¦ŗ󣬲śĪļŹĒNaOH”¢H2OŗĶ_________£»Ķعż__________²Ł×÷£ØĢīŠ“²Ł×÷Ćū³Ę£©£¬æÉŅŌŹ¹²śĪļĻą»„·ÖĄė”£
£Ø5£©ÉĻĶ¼ÖŠ£¬ÄÜŃ»·Ź¹ÓƵÄĪļÖŹŹĒC6H6”¢CaO”¢_________”¢_________”£
 ØDONa+CO2+H2O”ś
ØDONa+CO2+H2O”ś ØDOH+NaHCO3
ØDOH+NaHCO3½āĪö£ŗŹŌĢāµÄĒ°°ė²æ·Ö£¬Ö÷ŅŖÉę¼°·ĻĖ®ÖŠ±½·ÓµÄ“¦Ąķ·½·Ø”£×¢Ņā·ÖĪöŌŚ·ĻĖ®“¦Ąķ¹ż³ĢÖŠ±½·ÓµÄ“ęŌŚŠĪŹ½µÄ±ä»Æ£ŗ
±½·ÓŗĶĖ®µÄ»ģŗĻĪļ”ś±½·ÓŗĶ±½µÄ»ģŗĻĪļ”ś±½·ÓÄʵÄĖ®ČÜŅŗ”ś±½·ÓµÄĖ®ČÜŅŗ”ś±½·Ó
Ź×ĻČŌŚÉč±ø¢ńÖŠÓƱ½½«Ė®ČÜŅŗÖŠÅØ¶Č½ĻµĶµÄ±½·ÓŻĶČ”³öĄ“£¬ŌŚÉč±ø¢ņÖŠ½«±½·Ó×Ŗ»ÆĪŖ±½·ÓÄʶųŹµĻÖ±½·ÓÓė±½µÄ·ÖĄė£¬×īŗóŌŚÉč±ø¢óÖŠŌŁ½«±½·ÓÄĘ×Ŗ±äĪŖ³£ĪĀĻĀŌŚĖ®ČÜŅŗÖŠČܽā¶Č½ĻŠ”µÄ±½·Ó£¬²¢½«Ęä·ÖĄė³öĄ“”£½ųČėÉč±ø¢ōµÄÓ¦ŹĒ±½·ÓÓė¶žŃõ»ÆĢ¼·“Ó¦ŗóµÄ²śĪļĢ¼ĖįĒāÄĘ£¬Éč±ø¢ōÖŠ·¢ÉśµÄ·“Ó¦ĪŖCaO£«H2O£½Ca£ØOH£©2”¢NaHCO3+Ca£ØOH)2£½CaCO3”ż£«NaOH£«H2O”£¾¹żĀĖŗó£¬Éś³ÉµÄNaOHæÉŃ»·Ź¹ÓĆ£¬CaCO3ŌŚÉč±ø¢õÖŠ¾ģŃÉÕÓÖæÉ×Ŗ»ÆĪŖCaOŗĶCO2£¬½ų¶ųæÉŅŌ½ųŠŠŃ»·Ź¹ÓĆ”£


 Š”Ģģ²ÅæĪŹ±×÷ŅµĻµĮŠ“š°ø
Š”Ģģ²ÅæĪŹ±×÷ŅµĻµĮŠ“š°ø Ņ»æĪĖÄĮ·ĻµĮŠ“š°ø
Ņ»æĪĖÄĮ·ĻµĮŠ“š°ø »ĘøŌŠ”דŌŖĀś·Ö³å“ĢĪ¢²āŃéĻµĮŠ“š°ø
»ĘøŌŠ”דŌŖĀś·Ö³å“ĢĪ¢²āŃéĻµĮŠ“š°ø ŠĀøؽĢµ¼Ń§ĻµĮŠ“š°ø
ŠĀøؽĢµ¼Ń§ĻµĮŠ“š°ø Ńō¹āĶ¬Ń§Ņ»ĻßĆūŹ¦Č«ÓÅŗĆ¾ķĻµĮŠ“š°ø
Ńō¹āĶ¬Ń§Ņ»ĻßĆūŹ¦Č«ÓÅŗĆ¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
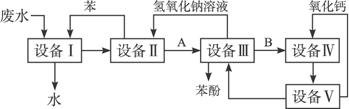
(1)ÉĻŹöĮ÷³ĢĄļ£¬Éč±ø¢ńÖŠ½ųŠŠµÄŹĒ²Ł×÷__________(ĢīŠ“²Ł×÷Ćū³Ę)”£ŹµŃéŹŅĄļÕāŅ»²½²Ł×÷æÉŅŌÓĆ__________½ųŠŠ(ĢīŠ“ŅĒĘ÷Ćū³Ę)”£
(2)ÓÉÉč±ø¢ņ½ųČėÉč±ø¢óµÄĪļÖŹAŹĒ__________£¬ÓÉÉč±ø¢ó½ųČėÉč±ø¢ōµÄĪļÖŹBŹĒ_________”£
(3)ŌŚÉč±ø¢óÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ__________”£
(4)ŌŚÉč±ø¢ōÖŠ£¬ĪļÖŹBµÄĖ®ČÜŅŗŗĶCaO·“Ó¦ŗ󣬲śĪļŹĒNaOH”¢H2OŗĶ__________”£Ķعż__________²Ł×÷(Ģī²Ł×÷Ćū³Ę)£¬æÉŅŌŹ¹²śĪļĻą»„·ÖĄė”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

£Ø1£©ÉĻŹöĮ÷³ĢĄļ£¬Éč±ø¢ńÖŠ½ųŠŠµÄŹĒ_________²Ł×÷£ØĢīŠ“²Ł×÷Ćū³Ę£©”£ŹµŃéŹŅĄļÕāŅ»²½²Ł×÷æÉŅŌÓĆ__________________£ØĢīŠ“ŅĒĘ÷Ćū³Ę£©½ųŠŠ”£
£Ø2£©ÓÉÉč±ø¢ņ½ųČėÉč±ø¢óµÄĪļÖŹAŹĒ_________”£ÓÉÉč±ø¢ó½ųČėÉč±ø¢ōµÄĪļÖŹBŹĒ_________”£
£Ø3£©ŌŚÉč±ø¢óÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ_______________________________________”£
£Ø4£©ŌŚÉč±ø¢ōÖŠ£¬ĪļÖŹBµÄĖ®ČÜŅŗŗĶCaO·“Ó¦ŗ󣬲śĪļŹĒNaOH”¢H2OŗĶ_________”£Ķعż²Ł×÷________________£ØĢīŠ“²Ł×÷Ćū³Ę£©£¬æÉŅŌŹ¹²śĪļĻą»„·ÖĄė”£
£Ø5£©Ķ¼ÖŠ£¬ÄÜŃ»·Ź¹ÓƵÄĪļÖŹŹĒC6H6”¢CaO”¢________________”¢________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ?
?
£Ø1£©ÉĻŹöĮ÷³ĢĄļ£¬Éč±ø¢ńÖŠ½ųŠŠµÄŹĒ””””””£ØĢīŠ“²Ł×÷Ćū³Ę£©²Ł×÷”£ŹµŃéŹŅĄļÕāŅ»²½²Ł×÷æÉŅŌÓĆ””””””””””£ØĢīŠ“ŅĒĘ÷Ćū³Ę£©½ųŠŠ”£
£Ø2£©ÓÉÉč±ø¢ņ½ųČėÉč±ø¢óµÄĪļÖŹAŹĒ””””””””””£¬ÓÉÉč±ø¢ó½ųČėÉč±ø¢ōµÄĪļÖŹBŹĒ”””””””””””£?
£Ø3£©ŌŚÉč±ø¢óÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ”””””””””””””£?
£Ø4£©ŌŚÉč±ø¢ōÖŠ£¬ĪļÖŹBµÄĖ®ČÜŅŗŗĶCaO·“Ó¦ŗ󣬲śĪļŹĒNaOH”¢H2OŗĶ”””””””£Ķعż””””””£ØĢīŠ“²Ł×÷Ćū³Ę£©””””””²Ł×÷£¬æÉŅŌŹ¹²śĪļĻą»„·ÖĄė”£?
£Ø5£©ÉĻĶ¼ÖŠ£¬ÄÜŃ»·Ź¹ÓƵÄĪļÖŹŹĒC6H6”¢CaO”¢?””””””””?”¢?””””””””?”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğÕć½Ź”ÓąŅ¦ÖŠŃ§ø߶žµŚŅ»“ĪÖŹĮæ¼ģ²ā»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗŹµŃéĢā
£Ø10·Ö£©ŗ¬±½·ÓµÄ¹¤Ņµ·ĻĖ®“¦ĄķµÄĮ÷³ĢĶ¼ČēĻĀ£ŗ
(1) ÉĻŹöĮ÷³ĢĄļ£¬Éč±ø¢ńÖŠ½ųŠŠµÄŹĒ_______________²Ł×÷(ĢīŠ“²Ł×÷Ćū³Ę)”£
(2) ÓÉÉč±ø¢ņ½ųČėÉč±ø¢óµÄĪļÖŹAŹĒ________”£ÓÉÉč±ø¢ó½ųČėÉč±ø¢ōµÄĪļÖŹBŹĒ________”£
(3) ŌŚÉč±ø¢óÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_________________________________________”£
(4) ŌŚÉč±ø¢ōÖŠ£¬ĪļÖŹBµÄĖ®ČÜŅŗŗĶCaO·“Ó¦ŗ󣬲śĪļŹĒNaOH”¢H2OŗĶ_________£¬Ķعż²Ł×÷_________ (ĢīŠ“²Ł×÷Ćū³Ę)æÉŅŌŹ¹²śĪļĻą»„·ÖĄė”£
(5) ÉĻĶ¼ÖŠÄÜŃ»·Ź¹ÓƵÄĪļÖŹŹĒC6H6”¢CaO”¢___________”¢___________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com