
����ʯ��������Ṥҵ��β��(��NO��NO2)��Ӧ�����ܾ���β�������ܻ��Ӧ�ù㷺��Ca(NO2)2���䲿�ֹ����������£�
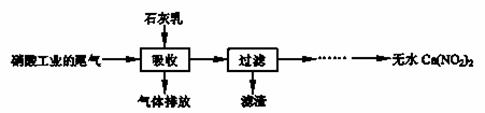
��ش��������⣺
��1��һ�������£�NO��NO2�������з�Ӧ��NO(g)��NO2(g)  N2O3(g)����ƽ�ⳣ������ʽΪK= ��
N2O3(g)����ƽ�ⳣ������ʽΪK= ��
��2�����������в�����Һ�����Ӵ�����(β�����������ײ����룬ʯ�������������������)����Ŀ���� ��������ѭ�����ã���������Ҫ�ɷ��� (�ѧʽ)��
��3���ù��������NO��NO2���ʵ���֮�Ƚӽ�1︰1����n(NO) ��n(NO2)��1︰1,��ᵼ�� ����n(NO) ��n(NO2)��1︰1,��ᵼ�� ��
��4����������Һ�豣�������ԣ���������Һ��Ca(NO2)2�ᷢ���ֽ⣬����֮һ��NO���䷴Ӧ�����ӷ���ʽ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ����д��ȷ���ǡ�������..��������������..��������.��..( )
A��ʵ�����ô���ʯ��ϡ������ȡCO2��2H+ + CO32- �� CO2��+ H2O
B������ϡ���ᷴӦ��Fe + 2H+ �� H 2��+ Fe 2+
C����AlCl3��Һ�м��������NaOH��Һ��Al3+ + 3OH- �� Al(OH)3��
D��NaHCO3��Һ��NaOH��Һ��Ӧ�� OH- + HCO3- �� CO32- + H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪����1��Zn��s��+ 1/2O2��g����ZnO(s) ����H��-350 kJ��mol-1 ��
��2��2Ag(s) + 1/2O2��g����Ag2O(s) ����H�� -25 kJ��mol-1 ��
��Zn��s��+ Ag2O(s)��ZnO(s)+ 2Ag(s)�Ħ�H����
A��-375 kJ��mol-1 B��-325 kJ��mol-1 C��+375 kJ��mol-1 D��+325 kJ��mol-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A����1��25��ʱ0.1mol��L��1HCl��Һ��pH��___________��
��2��25��ʱ0.1mol��L��1CH3COONa��Һ��pH____________7(�����������������)����ԭ���� �������ӷ���ʽ����ʾ����
��3������������Һ�������ϣ�����Ũ�ȴ�С˳����ȷ����_____________������ţ�
A��c(Na��)��c(Cl��)��c(OH��)��c(H��) B��c(Na��)��c(Cl��)��c(H��)��c(OH��)
C��c(Na��)��c(Cl��)��c(H��)��c(OH��) D��c(Cl��)��c(Na��)��c(OH��)��c(H��)
B����25���£���Ũ�Ⱦ�Ϊ0.1 mol��L��1��MgCl2��CuCl2�����Һ����μ��백ˮ��������
�������ѧʽ�������ɸó��������ӷ���ʽΪ ��
����֪25��ʱKsp[Mg(OH)2]��1.8��10-11��KsP[Cu(OH)2]��2.2��10-20��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ſ�ѧ�����IJ��Ͻ������о����ʵ��ֶκ�;��Խ��Խ�࣬N5����H3��O4��C60���ѱ����֡������й�˵���У���ȷ����
A��N5�� �����к���36������ B��H2��H3����ͬ��������
C��C60720 D��O2��O4����ͬλ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��Y��Z����Ԫ�ص�ԭ�ӣ������������Ų��ֱ�Ϊns1��2s22p3��2s22p4����������Ԫ����ɵij���������Ļ�ѧʽΪ
A��X2YZ3 B��X2YZ4 C��XYZ4 D��XYZ3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и������ʣ�ֻ��ˮ���Թܡ���ͷ�ιܾͿ��Լ������
A������ ������������ B���Ҵ�������������
C���ױ������Ȼ�̼�������� D���Ҵ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʽΪC5H7Cl���л����ṹ�������� �� ��
A��ֻ����1��˫����ֱ���л��� B����2��˫����ֱ���л���
C����1��˫���Ļ�״�л��� D����һ��������ֱ���л���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������绯ѧ��ʴ�ص��ǣ� ��
A�������ȴ�����������
B����ͭ��ͭп�Ͻ�������ͭ��ײ���ͭ��
C�����ʽ��¾��ú��ױ���䰵
D��������һ����ڳ�ˮ���°�һ������п��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com