
ijЩ��ѧ��Ӧ�ɱ�ʾΪ��A+B��C+D+H2O����ش��������⣺
��A��C��D��������Ԫ�أ���A����Ԫ�صĻ��ϼ۽���C��D֮�䣬��÷�Ӧ�����ӷ���ʽΪ_________________________________��
��AΪ��ɫ���壬C�ǻ���ɫ���壬��÷�Ӧ�����ӷ���ʽΪ__________________��
��AΪ�ڶ����ڵĵ��ʣ�BΪ�ڶ�����ijԪ������������ˮ���C��D��Ϊ���壬��÷�Ӧ�Ļ�ѧ����ʽΪ____________________________________��
��CΪNaCl��D����ʹ����ʯ��ˮ����ǵ���ɫ��ζ�����壬��A��______��_______��BΪ_____________(�ѧʽ����ͬ)��
��C��D������ʹ����ʯ��ˮ����ǵ����壬��A��B�����Ϊ_____________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
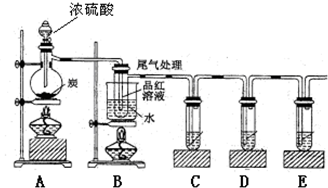
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�긣��ʡ����ͩ��ѧ������ѧ������������ѧ�Ծ��������棩 ���ͣ������
ijЩ��ѧ��Ӧ�ɱ�ʾΪ��A+B��C+D+H2O����ش��������⣺
��1����A��C��D��������Ԫ�أ���A����Ԫ�صĻ��ϼ۽���C��D֮�䣬��÷�Ӧ���ӷ���ʽΪ____________________________________________________��
��2����AΪ��ɫ���壬C�ǻ���ɫ���壬��÷�Ӧ�Ļ�ѧ����ʽΪ______________________��
��3����CΪNaCl��D����ʹ����ʯ��ˮ����ǵ���ɫ��ζ�����壬��AΪ�Σ�BΪ�ᣬ��A��___________________��___________________ (�ѧʽ)��
��4����A������һ�������B�����ᣬ��A��B��Ӧ�Ļ�ѧ����ʽΪ___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���㽭ʡ�����и�����ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��12�֣���.ijЩ��ѧ��Ӧ�ɱ�ʾΪ��A+B��C+D+H2O����ش��������⣺
��1����A��C��D��������Ԫ�أ���A����Ԫ�صĻ��ϼ۽���C��D֮�䣬�÷�Ӧ���ӷ���ʽΪ____________ ________��
��2����AΪ��ɫ���壬C��D��Ϊ���壬��÷�Ӧ�Ļ�ѧ����ʽΪ_____ ___��
��3����CΪNaCl��D����ʹ����ʯ��ˮ����ǵ���ɫ�д̼�����ζ�����壬��AΪ�Σ�BΪ�ᣬ��A�� ��__ ____(�ѧʽ)��
��4����A������һ�������B�����ᣬ��A��B��Ӧ�Ļ�ѧ����ʽΪ_ ___��
��.ijЩ��ѧ��Ӧ�ɱ�ʾΪ��A+B +H2O��C+D����ش��������⣺
����Ӧ�����������и���һ�����ʺ���SiԪ�أ���д��2������Ҫ��Ļ�ѧ��Ӧ����ʽ
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꽭��ʡ������ѧ�ڵ������¿���ѧ�Ծ� ���ͣ�ʵ����
ijЩ��ѧ��Ӧ�ɱ�ʾΪ��A+B��C+D+H2O����ش��������⣺
��1����A��C��D��������Ԫ�أ���A����Ԫ�صĻ��ϼ۽���C��D֮�䣬��÷�Ӧ�����ӷ���ʽΪ_________________________________��
��2����AΪ��ɫ���壬C�ǻ���ɫ���壬��÷�Ӧ�����ӷ���ʽΪ__________________��
��3����AΪ�ڶ����ڵĵ��ʣ�BΪ�ڶ�����ijԪ������������ˮ���C��D��Ϊ���壬��÷�Ӧ�Ļ�ѧ����ʽΪ____________________________________��
��4����CΪNaCl��D����ʹ����ʯ��ˮ����ǵ���ɫ��ζ�����壬��A��______��_______��BΪ_____________(�ѧʽ����ͬ)��
��5����C��D������ʹ����ʯ��ˮ����ǵ����壬��A��B�����Ϊ_____________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com