
ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø £©
A. S2-µē×ÓÅŲ¼Ź½ 1s22s22p63s23p4
B. ŌŚ½šŹō¾§ĢåÖŠ£¬×ŌÓɵē×ÓÓė½šŹōĄė×Ó»ņ½šŹōŌ×ÓµÄÅöײӊÄÜĮæ“«µŻ£¬æÉŅŌÓĆ“ĖĄ“½āŹĶµÄ½šŹōµÄĪļĄķŠŌÖŹŹĒµ¼ČČŠŌ”£
C. ½šŹō¼üæÉŅŌæ“×öŹĒŠķ¶ąŌ×Ó¹²ÓĆŠķ¶ąµē×ÓĖłŠĪ³ÉµÄĒæĮŅĻą»„×÷ÓĆ£¬ĖłŅŌŗĶ¹²¼Ū¼üĄąĖĘ£¬Ņ²ÓŠ±„ŗĶŠŌŗĶ·½ĻņŠŌ”£
D. ijĪļÖŹµÄ¾§ĢåÖŠŗ¬A”¢B”¢CČżÖÖŌŖĖŲ£¬ĘäÅÅĮŠ·½Ź½ČēĶ¼ĖłŹ¾£¬¾§°ūÖŠA”¢B”¢CµÄŌ×ÓøöŹż±ČĪŖ1”Ć2”Ć2”£

 ½ĢѧĮ·ŠĀĶ¬²½Į·Ļ°ĻµĮŠ“š°ø
½ĢѧĮ·ŠĀĶ¬²½Į·Ļ°ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
»ÆѧÓĆÓļŹĒ»ÆѧѧæʵÄĢŲÉ«ÓļŃŌ£¬»ÆѧÓĆÓļæÉŅŌ×¼Č·±ķŹö»ÆѧĻÖĻ󔢱ä»ÆŅŌ¼°±¾ÖŹ”£Ķź³ÉĻĀĮŠÓŠ¹Ų·½³ĢŹ½”£
£Ø1£©Na2S2O3»¹ŌŠŌ½ĻĒ棬ŌŚČÜŅŗÖŠŅ×±»Cl2Ńõ»Æ³ÉSO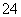 £¬³£ÓĆ×÷ĶŃĀČ¼Į£¬øĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ______________________________________________________£»
£¬³£ÓĆ×÷ĶŃĀČ¼Į£¬øĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ______________________________________________________£»
£Ø2£©ĖįŠŌøßĆĢĖį¼ŲČÜŅŗÓė¹żŃõ»ÆĒāČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ
________________________________________________________________________ӣ
£Ø3£©»Æѧ·“Ó¦¶ą×Ė¶ą²Ź£¬°ŃSO2ĶØČėĻõĖįĢśČÜŅŗÖŠ£¬ČÜŅŗÓÉ×Ų»ĘÉ«±äĪŖĒ³ĀĢÉ«£¬µ«Į¢¼“ÓÖ±äĪŖ×Ų»ĘÉ«£¬“ĖŹ±ĻņČÜŅŗÖŠµĪ¼ÓĀČ»Æ±µČÜŅŗ£¬ÓŠ°×É«³Įµķ²śÉś”£ĒėŠ“³öÉĻŹö±ä»ÆÖŠČÜŅŗÓÉ×Ų»ĘÉ«±äĪŖĒ³ĀĢÉ«£¬µ«Į¢¼“ÓÖ±äĪŖ×Ų»ĘÉ«ĖłÉę¼°µÄĮ½øöĄė×Ó·½³ĢŹ½£ŗ
________________________________Ӣ________________________________________ӣ
£Ø4£©Ļņŗ¬ÓŠn mol äå»ÆŃĒĢśµÄČÜŅŗÖŠĶØČėµČĪļÖŹµÄĮæµÄµÄĀČĘų£¬ĒėŠ“³öĄė×Ó·½³ĢŹ½£ŗ
_________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖX”¢YŌŖĖŲĶ¬ÖÜĘŚ£¬ĒŅµēøŗŠŌX£¾Y£¬ĻĀĮŠĖµ·Ø“ķĪóµÄŹĒ
A£®XÓėYŠĪ³É»ÆŗĻĪļŹĒ£¬XæÉŅŌĻŌøŗ¼Ū£¬YĻŌÕż¼Ū
B£®µŚŅ»µēĄėÄÜæÉÄÜYŠ”ÓŚX
C£®×īøß¼Ūŗ¬ŃõĖįµÄĖįŠŌ£ŗX¶ŌÓ¦µÄĖįŠŌČõÓŚÓŚY¶ŌÓ¦µÄĖįŠŌ
D£®ĘųĢ¬Ēā»ÆĪļµÄĪČ¶ØŠŌ£ŗHmYŠ”ÓŚHnX
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĶ¼Ź½±ķŹ¾ÕżČ·µÄŹĒ
A£®¶žŃõ»ÆĢ¼  B.NŌ×ӵļŪµē×Óµē×ÓÅŲ¼Ķ¼ £ŗ
B.NŌ×ӵļŪµē×Óµē×ÓÅŲ¼Ķ¼ £ŗ 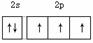
 C£®ĀĮĄė×ӵĽį¹¹Ź¾ŅāĶ¼ D£®ŅŅ“¼µÄ·Ö×ÓŹ½£ŗC2H5OH
C£®ĀĮĄė×ӵĽį¹¹Ź¾ŅāĶ¼ D£®ŅŅ“¼µÄ·Ö×ÓŹ½£ŗC2H5OH
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓĆVSEPR ĄķĀŪÅŠ¶Ļ£ŗ
| ĪļÖŹ | ¼Ū²ćµē×Ó¶ŌŹż | ¹ģµĄŌӻƊĪŹ½ | ·Ö×Ó»ņĄė×ÓµÄĮ¢ĢåŠĪד | ¼ü½Ē |
| COCl2 | ||||
| H3O+ | ||||
| CS2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĖµ·Ø“ķĪóµÄŹĒ£Ø £©
A£®ŅŃÖŖ2NO2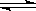 N2O4¼ÓŃ¹ŗóŃÕÉ«ĻȱäÉīŗó±äĒ³”£
N2O4¼ÓŃ¹ŗóŃÕÉ«ĻȱäÉīŗó±äĒ³”£
B. ŌŚĆܱÕČŻÖŠ·¢ÉśĻĀĮŠ·“Ó¦aA(g) cC(g)£«dD(g)£¬·“Ó¦“ļµ½Ę½ŗāŗ󣬽«ĘųĢåĢå»żŃ¹Ėõµ½ŌĄ“µÄŅ»°ė£¬µ±ŌŁ“Ī“ļµ½Ę½ŗāŹ±£¬DµÄÅضČĪŖŌĘ½ŗāµÄ1.6±¶£¬¹ŹæÉÖŖAµÄ×Ŗ»ÆĀŹ±äŠ””£
cC(g)£«dD(g)£¬·“Ó¦“ļµ½Ę½ŗāŗ󣬽«ĘųĢåĢå»żŃ¹Ėõµ½ŌĄ“µÄŅ»°ė£¬µ±ŌŁ“Ī“ļµ½Ę½ŗāŹ±£¬DµÄÅضČĪŖŌĘ½ŗāµÄ1.6±¶£¬¹ŹæÉÖŖAµÄ×Ŗ»ÆĀŹ±äŠ””£
C£®ŌŚĆܱÕČŻĘ÷ÖŠ½ųŠŠČēĻĀ·“Ó¦£ŗX2(g)+Y2(g)  2Z(g)£¬ŅŃÖŖX2”¢Y2”¢ZµÄĘšŹ¼ÅØ¶Č·Ö±šĪŖ0.1mol/L”¢0.3mol/L”¢0.2mol/L£¬ŌŚŅ»¶ØĢõ¼žĻĀ£¬µ±·“Ó¦“ļµ½Ę½ŗāŹ±£¬ZµÄÅضČÓŠæÉÄÜŹĒ0.4 mol/L”£
2Z(g)£¬ŅŃÖŖX2”¢Y2”¢ZµÄĘšŹ¼ÅØ¶Č·Ö±šĪŖ0.1mol/L”¢0.3mol/L”¢0.2mol/L£¬ŌŚŅ»¶ØĢõ¼žĻĀ£¬µ±·“Ó¦“ļµ½Ę½ŗāŹ±£¬ZµÄÅضČÓŠæÉÄÜŹĒ0.4 mol/L”£
D. Ī“Ą“ŠĀÄÜŌ“µÄĢŲµćŹĒ׏Ō“·įø»£¬ŌŚŹ¹ÓĆŹ±¶Ō»·¾³ĪŽĪŪČ¾»ņĪŪČ¾ŗÜŠ”£¬ĒŅæÉŅŌŌŁÉś”£ĻĀĮŠŹōÓŚĪ“Ą“ŠĀÄÜŌ“±ź×¼µÄŹĒ¢Ż¢Ž¢ß¢ą”£
¢ŁĢģČ»Ęų ¢ŚĆŗ ¢ŪŗĖÄÜ ¢ÜŹÆÓĶ ¢ŻĢ«ŃōÄÜ ¢ŽÉśĪļÖŹÄÜ ¢ß·ēÄÜ ¢ąĒāÄÜ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÖŠŗĶpHĻąĶ¬”¢Ģå»żĻąĶ¬µÄH2SO4 ”¢HNO3 ŗĶCH3COOHČÜŅŗ£¬ĻūŗÄĶ¬Ņ»ÅØ¶ČµÄNaOHČÜŅŗ£¬Ģå»ż·Ö±šĪŖV1”¢V2ŗĶV3 £¬ŌņV1”¢V2ŗĶV3 µÄ¹ŲĻµÕżČ·µÄŹĒ
A£®V1£¾V2£½V3 B£®V3 £¾V2£½V1 C£®V1£¾V2£¾V3 D£®V1£½V2£½V3
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
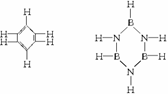
ŅŃÖŖ»ÆŗĻĪļB3N3H6(ÅšµŖ±½)ÓėC6H6(±½)µÄ·Ö×Ó½į¹¹ĻąĖĘ£¬
ŌņÅšµŖ±½µÄ¶žĀČČ”“śĪļB3N3H4Cl2µÄĶ¬·ÖŅģ¹¹ĢåµÄŹżÄæĪŖ
A.2ÖÖ B.3ÖÖ C.4ÖÖ D.6ÖÖ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
·śŌŖĖŲ°ė¾¶Š”£¬µēøŗŠŌøߣ¬æÉÓė½šŹō»ņ·Ē½šŹōÉś³É×īøß¼Ū»ÆŗĻĪļ£¬ČēMnF2”¢SF6µČ”£
£Ø1£©Š“³öF-µÄµē×ÓÅŲ¼Ź½ ”£
£Ø2£©BF3µÄ¼ü½ĒŹĒ £¬BF3æÉÓėNaF·“Ӧɜ³ÉNaBF4£¬Š“³öBF4-µÄ½į¹¹Ź½ ”£
£Ø3£©OF2æÉÓɵ„ÖŹ·śÓėĻ”NaOHČÜŅŗ·“Ó¦Ą“Öʱø”£
¢ŁOF2·Ö×ÓµÄæռ乹ŠĶĪŖ ”£
¢ŚOF2æÉÓėĖ®»ŗĀż·“Ó¦£¬Ęä·½³ĢŹ½ĪŖ £»Cl2OÓėOF2»„ĪŖµČµē×ÓĢ壬ŹŌŠ“³öCl2OÓėĖ®·“Ó¦µÄ·½³ĢŹ½£ŗ ”£
¢ŪŹŌ±Č½ĻOF2ÓėCl2O¼«ŠŌµÄ“󊔣¬²¢¼ņµ„ĖµĆ÷ŌŅņ£ŗ
ӣ
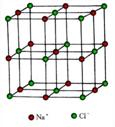 £Ø4£©NaF¾ßÓŠÓėNaClĻąĶ¬µÄ¾§ĢåĄąŠĶ£¬ŌņF-ÖÜĪ§¾ąĄė×ī½üµÄNa+ĖłĪ§³ÉµÄ¼øŗĪĢåĪŖ £¬NaFÖŠNa+ÓėNa+Ö®¼äµÄ×ī¶Ģ¾ąĄėĪŖd£¬ŌņNaF¾§ĢåµÄĆܶČĪŖ £Ø°¢·üŁ¤µĀĀŽ³£ŹżÓĆNA±ķŹ¾£¬ĮŠ³ö¼ĘĖćŹ½£©”£
£Ø4£©NaF¾ßÓŠÓėNaClĻąĶ¬µÄ¾§ĢåĄąŠĶ£¬ŌņF-ÖÜĪ§¾ąĄė×ī½üµÄNa+ĖłĪ§³ÉµÄ¼øŗĪĢåĪŖ £¬NaFÖŠNa+ÓėNa+Ö®¼äµÄ×ī¶Ģ¾ąĄėĪŖd£¬ŌņNaF¾§ĢåµÄĆܶČĪŖ £Ø°¢·üŁ¤µĀĀŽ³£ŹżÓĆNA±ķŹ¾£¬ĮŠ³ö¼ĘĖćŹ½£©”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com