
| 100mL��1.06g/mL��20% |
| 106g/mol |


 ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д� �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���Ȼ�����Һ�м��������ˮ��Al3++4NH3?H2O=AlO2-+4NH4++2H2O |
| B��NaHCO3��Һ������Ba��OH��2��Һ��ϣ�HCO3-+OH-+Ba2+�TH2O+BaCO3�� |
| C��NaHCO3��Һ����Ba��OH��2��Һ��ϣ�2HCO3-+2OH-+Ba2+�TBaCO3��+2H2O+CO32- |
| D��NaAlO2��Һ��ͨ������CO2��2A1O2-+CO2+3H2O�T2Al��OH��3��+CO32- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
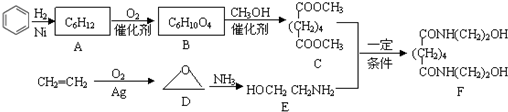
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
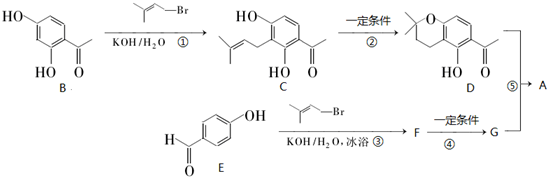
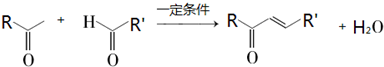
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
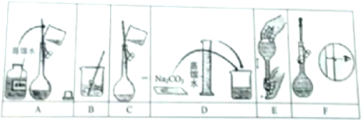
 ij��ѧ��ȤС���ͬѧΪ̽����������Ļ�ѧ���ʣ��������ͼ��ʾ��װ�ã�
ij��ѧ��ȤС���ͬѧΪ̽����������Ļ�ѧ���ʣ��������ͼ��ʾ��װ�ã��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���Ʊ�����ú���� |
| B����ˮ�ɳ��ڱ�������ɫƿ�� |
| C����������Ӧ�ܷⱣ�� |
| D��Ư�۲�Ӧ�����ڿ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com