
����Ŀ��A��B��C�����ֳ���������Ԫ�صĵ��ʡ�������DΪ��ɫҺ�壬E��һ�ֳ������������塣��ת����ϵ��ͼ(��Ӧ�����Ͳ��ֲ�����ȥ)���Իش�
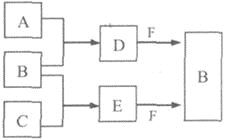
(1)E�ĵ���ʽ��__________��
(2)����X��B��D���ܷ�Ӧ���ɺ�ɫ����Y��Y�Ļ�ѧʽ��_________��
(3)����Z�����ڶԿ�������ɱ����������ˮ�����ʵȡ�Z��B�����Ԫ����ͬ��Z�����и�ԭ������������֮��Ϊ18��Z�����Ե⻯����Һ��Ӧ����B�͵ⵥ�ʣ���Ӧ�����ӷ���ʽ��__________________________��
(4)ȡ0.3 mol F������D��ֻ�Ϻ�������Һ����ͨ��0.2 mol E��ַ�Ӧ�����õ���ˮ��Һ�и������ӵ�Ũ���ɴ�С��˳����(������H+)_________________________��
���𰸡�![]()
![]()
![]()
![]()
��������
�ƶ���Ҫȷץסͻ�ƿڣ�������DΪ��ɫҺ�壬����֪��D��H2O����E��һ�ֳ���������������E��CO2����ΪA��B��C�ǵ��ʣ���A��H2��BΪO2��C��̼��H2O��CO2��������Na2O2����O2������FΪNa2O2������������ˮ�������ɺ�ɫ����ģ���Ӧ���������������������������������Ԫ����ͬ�ģ�����������֮��Ϊ18���dz��������������Ե⻯�ط�Ӧע�⻯�ϼ�������Ƚ�����ƽ��0.3 mol Na2O2��������ˮ��Ӧ����0.6mol��NaOH,��0.2mol��CO2��Ӧ,�õ�0.2mol��Na2CO3��ʣ��0.2molNaOH����ΪCO32��ˮ�⣬������Ũ�ȵĴ�С��![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��������ʵ��װ��һ������ʲôʵ���������ʵ��������ƣ�
A __________ B ___________ C ___________
A��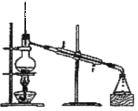 B��
B��![]() C��
C��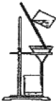
����3p%��������ͬ�����p%�������ϵõ�q%��ϡ���ᣬ��p��q�Ĺ�ϵ��ȷ����______��
��2����ͬ�¡�ͬѹ�£�ʵ����CO��N2��SO2��������Ļ��������ܶ���H2��18.5��������SO2����������Ϊ ________��������CO��N2�����ʵ���֮��Ϊ1��1��������������Ԫ�ص���������Ϊ ________����С�������1λ��
��3����ͬ�����£�ijCl2��O2�������200 mLǡ����300 mL H2��������HCl��H2O����֪��H2+Cl2=2HCl��������������Cl2��O2�������Ϊ_____����������ƽ��Ħ������Ϊ_______________����С�������1λ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����.����غ�Ũ����֮�������з�Ӧ��2KClO3+4HCl(Ũ)=2KCl+Cl2��+2ClO2��+2H2O.
��1���÷�Ӧ�з�����ԭ��Ӧ��������________������������________��
��2����˫���ű������ʽ�еĵ��ӵ�ʧ2KClO3+4HCl(Ũ)=2KCl+Cl2��+2ClO2��+2H2O________
��3������0.2 mol���ӷ���ת��ʱ�����ɵ����������Ϊ________L (��״��)����������HCl�����ʵ���Ϊ________mol.
��4�����ֱ��â�KMnO4(��ԭ������Mn2+)��MnO2(��ԭ������Mn2+) ��Ca(ClO)2(��ԭ������Cl2)����Ũ�����Ʊ���������Ũ���������������������������ʵ�����ͬʱ���������������ʵ������ٵ���________��
��.��֪��Ӧ��Cu+HNO3(ϡ)����Cu(NO3)2+NO��+H2O��
��1����ƽ������˫���ŷ�����ʾ������Ӧ�е���ת�Ƶķ������Ŀ��___Cu+___HNO3(ϡ) �� ___Cu(NO3)2+___NO��+___H2O��___________
��2���÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ_______��д���÷�Ӧ�����ӷ���ʽ______________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ԫ��R��T��Q��W��Ԫ�����ڱ��е����λ����ͼ��ʾ������T������������������������ȡ������жϲ���ȷ���ǣ� ��

A. �����̬�⻯������ȶ��ԣ�R��Q
B. ����������Ӧˮ��������ԣ�Q��W
C. ԭ�Ӱ뾶��T��Q��R
D. ����T��NaOH��Һ����Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ������Fe��FeO��Fe2O3�Ļ�����У�����500 mL 1 mol/L�����ᣬǡ��ʹ�������ȫ�ܽ⣬�ų�672 mL(��״��)���塣��������Һ�м���KSCN��Һ��Ѫ��ɫ���֣���ô����������CO�ڸ����»�ԭ��ͬ�����Ĵ˻����ܵõ���(����)
A. 28 g B. 14 g
C. 12.32 g D. �������㣬������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪A��B��F�Ǽ�ͥ�г������л��E��ʯ�ͻ�����չˮƽ�ı�־��F��һ�ֳ����ĸ߷��Ӳ��ϡ���������ת����ϵ�ش��������⣺

(1)�����ޡ������ߵ����Ʒֱ�Ϊ________��________��
(2)���������зе���ߵ���________��
A ���� B ú��
C ���� D ����
(3)�ڢ١���������ȡ����Ӧ����________��ԭ��������Ϊ100%�ķ�Ӧ��________��(�����)
(4)д���ṹ��ʽ��A________��F________��
(5)д����Ӧ�۵����ӷ���ʽ��___________��
(6)��Ϊ��ͥ�г���������F���������Ǵ����˼���ķ��㣬ͬʱҲ����˻�����Ⱦ��������Ⱦ��Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��[�л���ѧ����]
���л���B��صķ�Ӧ��Ϣ���£�
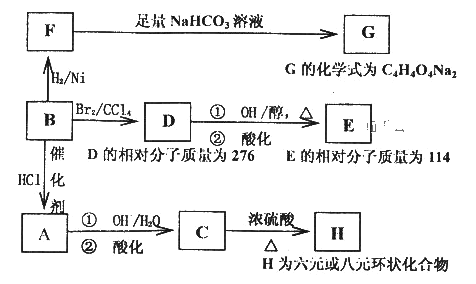
(1)д�����з�Ӧ�ķ�Ӧ���ͣ�
B��A______________��D��E�ڢٲ���Ӧ________________��
(2)д��E��C�Ľṹ��ʽ��E_______________��C_______________��
(3)C��һ��ͬ���칹��K�������ص㣺1molK���Ժ�3mol�����Ʒ�����Ӧ�ų�33��6LH2(��״����)��1molK���Ժ�������NaHCO3��Һ��Ӧ������1molCO2��1molK�����Է���������Ӧ����2molAg����д��K�Ľṹ��ʽ________________________��
(4)д����Ũ������ڲ����ȵ������£�F�������Ҵ���Ӧ�Ļ�ѧ����ʽ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ������������ͨ���ۺ�ˮ������Ӧ�����Եõ�������������������ֿ��Ծ����˷�Ӧ���淴Ӧ�����ɿ�����ϸ�����ۣ��������۾��кܸߵķ�Ӧ���ԣ��ڿ�������ײ��������ʱ��ȼ�գ������׳ơ�������������ֱ�����ͼ��ʾ�����������װ�ã���ȡ��������������͡�����������ʵ���б���ʹ����ͨ���ۺ�6 mol��L��1���ᣬ�����Լ���ѡ(װ���б�Ҫ������̨�����С���Ȧ��ʯ�����������豸����ͼ�о�����ȥ)����д���пհף�
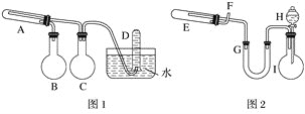
(1)ʵ�����ʱ�Թ�A��Ӧ������Լ���__________����ƿB��������____________����ƿC��������__________�����Թ�D���ռ��õ�����__________��
(2)ʵ��ʱ��U�ι�G��Ӧ������Լ���____________������©��H��Ӧ����____________��
(3)����װ���У���ʵ��ʱ��Ҫ���ȵ�������(���������Ӧ����ĸ)__________________��
(4)�Թ�E�з�����Ӧ�Ļ�ѧ����ʽ��__________________________��
(5)Ϊ�˰�ȫ����E���еķ�Ӧ����ǰ����F���ڴ�����____________��E���еķ�Ӧ��ʼ����F���ڴ�Ӧ________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��ʱ��CH3COOH�ĵ��볣��Ka=1.8��10��5�������£���25mL�������Ʊ���Һ����μ���0.1mol��L��1��CH3COOH��Һ��pH�仯������ͼ��ʾ��

��1��������������Һ�����ʵ���Ũ��Ϊ__��
��2��A���Ӧ�ĺ�����Ϊ25mL���������ӷ���ʽ����A����ʾ����Һ�Լ��Ե�ԭ��__��
��3��A����ʾ����Һ�и�����Ũ���ɴ�С������˳���ǣ�______��
��4��B����ʾ��Һ�У�![]() ��___��
��___��
��5��C����ʾ��ҺΪ��Ũ�ȵ�CH3COONa��CH3COOH�����Һ�����жϸ���Һ��c(CH3COOH)__c(CH3COO��)��(������������������������)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com