
·ÖĪö £Ø1£©ČōA”¢DµÄĖ®ČÜŅŗ¾łÄÜŹ¹ŹŖČóµÄĄ¶É«ŹÆČļŹŌÖ½±äŗģ£¬ĖµĆ÷ČÜŅŗ³ŹĖįŠŌ£¬BŌŚ³£ĪĀŹ±ĪŖĘųĢ壬ŌņAĪŖH2S£¬BĪŖSO2£¬CĪŖSO3£¬DĪŖH2SO4£»
£Ø2£©ČōAµÄĖ®ČÜŅŗÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶£¬ŌņAŹĒNH3£¬DµÄĻ”ČÜŅŗÄÜŹ¹ŹŖČóµÄĄ¶É«ŹÆČļŹŌÖ½±äŗģ£¬DŹōÓŚĖį£¬°±Ęų±»Ńõ»ÆÉś³ÉNO£¬NO±»ŃõĘųŃõ»ÆÉś³ÉNO2£¬ĖłŅŌBŹĒNO”¢CŹĒNO2”¢DŹĒHNO3£®
½ā“š ½ā£ŗ£Ø1£©ČōA”¢DµÄĖ®ČÜŅŗ¾łÄÜŹ¹ŹŖČóµÄĄ¶É«ŹÆČļŹŌÖ½±äŗģ£¬ĖµĆ÷ČÜŅŗ³ŹĖįŠŌ£¬BŌŚ³£ĪĀŹ±ĪŖĘųĢ壬ŌņAĪŖH2S£¬BĪŖSO2£¬CĪŖSO3£¬DĪŖH2SO4£¬B”śC×Ŗ»ÆµÄ»Æѧ·“Ó¦·½³ĢŹ½£ŗ2SO2+O2$?_{”÷}^{“߻ƼĮ}$2SO3£¬
¹Ź“š°øĪŖ£ŗH2S£»2SO2+O2$?_{”÷}^{“߻ƼĮ}$2SO3£»
£Ø2£©ČōAµÄĖ®ČÜŅŗÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶£¬ŌņAŹĒNH3£¬DµÄĻ”ČÜŅŗÄÜŹ¹ŹŖČóµÄĄ¶É«ŹÆČļŹŌÖ½±äŗģ£¬DŹōÓŚĖį£¬°±Ęų±»Ńõ»ÆÉś³ÉNO£¬NO±»ŃõĘųŃõ»ÆÉś³ÉNO2£¬ĖłŅŌBŹĒNO”¢CŹĒNO2”¢DŹĒHNO3£®
¢ŁAµÄ»ÆѧŹ½ĪŖ£ŗNH3£¬ŹµŃéŹŅÖʱøĘųĢåAµÄ»Æѧ·“Ó¦·½³ĢŹ½ĪŖ£ŗ2NH4Cl+Ca£ØOH£©2$\frac{\underline{\;\;”÷\;\;}}{\;}$2NH3”ü+CaCl2+2H2O£¬A”śB×Ŗ»ÆµÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ4NH3+5O2$\frac{\underline{“߻ƼĮ}}{”÷}$NO+6H2O£¬
¹Ź“š°øĪŖ£ŗNH3£»2NH4Cl+Ca£ØOH£©2$\frac{\underline{\;\;”÷\;\;}}{\;}$2NH3”ü+CaCl2+2H2O£»4NH3+5O2$\frac{\underline{“߻ƼĮ}}{”÷}$NO+6H2O£»
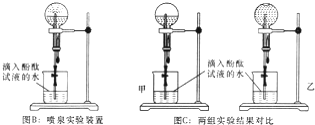
¢Śøł¾ŻĶ¼BÅēČŖŹµŃéµÄ×°ÖĆ½ųŠŠŹµŃ飬ÄܹŪ²ģµ½ĆĄĄöµÄŗģÉ«ÅēČŖ£¬ÓĆ·½³ĢŹ½½āŹĶÅēČŖ³ŹŗģÉ«µÄŌŅņ£ŗNH3+H2O?NH3£®H2O?NH4++OH-£¬
¹Ź“š°øĪŖ£ŗNH3+H2O?NH3£®H2O?NH4++OH-£»
¢ŪČÜŅŗĢå»żµČÓŚ°±ĘųµÄĢå»ż£¬¼××éĶ¬Ń§ĖłµĆČÜŅŗµÄĪļÖŹµÄĮæÅØ¶ČµČÓŚŅŅ×éĶ¬Ń§ĖłµĆČÜŅŗµÄĪļÖŹµÄĮæÅØ¶Č£¬
¹Ź“š°øĪŖ£ŗµČÓŚ£®
µćĘĄ ±¾Ģāæ¼²éĪŽ»śĪļĶʶĻ£¬øł¾ŻĪļÖŹµÄŃÕÉ«”¢ĪļÖŹµÄŠŌÖŹ½ųŠŠĶʶĻ£¬ŹģĮ·ÕĘĪÕ֊ѧ³£¼ūĮ¬Šų·“Ó¦£¬ÄѶČÖŠµČ£®


 æģĄÖ5¼Ó2½š¾ķĻµĮŠ“š°ø
æģĄÖ5¼Ó2½š¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | CCl4”¢SiCl4”¢SiH4 | B£® | H2S”¢NF3”¢CH4 | C£® | BCl3”¢NH3”¢CO2 | D£® | SO3”¢BF3”¢H3O+ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
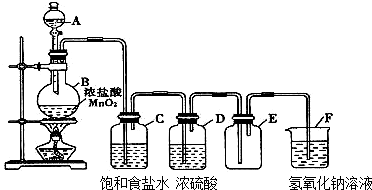
 ČŪČŚŃĪČ¼ĮĻµē³Ų¾ßÓŠ½Ļøߵķ¢µēŠ§ĀŹ£¬Ņņ¶ųŹÜµ½ÖŲŹÓ£®Ä³Č¼ĮĻµē³ŲŅŌČŪČŚµÄK2CO3ĪŖµē½āÖŹ£¬ŅŌ¶”Ķé£ØC4H10£©ĪŖČ¼ĮĻ£¬ŅŌæÕĘųĪŖŃõ»Æ¼Į£¬ŅŌ¾ßÓŠ“ß»Æ×÷ÓĆŗĶµ¼µēŠŌÄܵÄĻ”ĶĮ½šŹō²ÄĮĻĪŖµē¼«£®øĆČ¼ĮĻµē³Ųøŗ¼«µē¼«·“Ó¦Ź½ĪŖ2C4H10+26CO${\;}_{3}^{2-}$-52e-ØT34CO2+10H2O£®
ČŪČŚŃĪČ¼ĮĻµē³Ų¾ßÓŠ½Ļøߵķ¢µēŠ§ĀŹ£¬Ņņ¶ųŹÜµ½ÖŲŹÓ£®Ä³Č¼ĮĻµē³ŲŅŌČŪČŚµÄK2CO3ĪŖµē½āÖŹ£¬ŅŌ¶”Ķé£ØC4H10£©ĪŖČ¼ĮĻ£¬ŅŌæÕĘųĪŖŃõ»Æ¼Į£¬ŅŌ¾ßÓŠ“ß»Æ×÷ÓĆŗĶµ¼µēŠŌÄܵÄĻ”ĶĮ½šŹō²ÄĮĻĪŖµē¼«£®øĆČ¼ĮĻµē³Ųøŗ¼«µē¼«·“Ó¦Ź½ĪŖ2C4H10+26CO${\;}_{3}^{2-}$-52e-ØT34CO2+10H2O£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
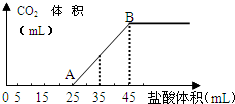 ½«Ņ»¶ØÖŹĮæµÄĢ¼ĖįÄĘŗĶĒāŃõ»ÆÄĘµÄ¹ĢĢå»ģŗĻĪļĶźČ«ČܽāÓŚĖ®£¬ÖĘ³ÉĻ”ČÜŅŗ£¬Č»ŗóĻņøĆČÜŅŗÖŠÖšµĪ¼ÓČė1mol•L-1µÄŃĪĖį£¬Ėł¼ÓČėŃĪĖįµÄĢå»żÓė²śÉśCO2µÄĢå»ż£Ø±ź×¼×“æö£©¹ŲĻµČēĶ¼ĖłŹ¾£ŗ
½«Ņ»¶ØÖŹĮæµÄĢ¼ĖįÄĘŗĶĒāŃõ»ÆÄĘµÄ¹ĢĢå»ģŗĻĪļĶźČ«ČܽāÓŚĖ®£¬ÖĘ³ÉĻ”ČÜŅŗ£¬Č»ŗóĻņøĆČÜŅŗÖŠÖšµĪ¼ÓČė1mol•L-1µÄŃĪĖį£¬Ėł¼ÓČėŃĪĖįµÄĢå»żÓė²śÉśCO2µÄĢå»ż£Ø±ź×¼×“æö£©¹ŲĻµČēĶ¼ĖłŹ¾£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā



 +2H2O£®
+2H2O£®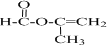 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¼×ĶéµÄČ¼ÉÕ | B£® | Ō×Ó½įŗĻ³É·Ö×Ó | ||
| C£® | ·Ö½ā·“Ó¦ | D£® | ×ĘČȵÄĢ¼Óė¶žŃõ»ÆĢ¼·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | Ę·ŗģČÜŅŗ | B£® | ĖįŠŌøßĆĢĖį¼ŲČÜŅŗ | ||
| C£® | äåĖ® | D£® | ³ĪĒåµÄŹÆ»ŅĖ® |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com