
ĻÖÓŠ25 ”ꏱ0.1 mol”¤L£1µÄ°±Ė®£¬Ēė»Ų“šŅŌĻĀĪŹĢā£ŗ
£Ø1£©ČōĻņ°±Ė®ÖŠ¼ÓČėÉŁĮæĮņĖįļ§¹ĢĢ壬Ņ»Ė®ŗĻ°±µÄµēĄėĘ½ŗā________(Ģī”°Ļņ×ó”±”¢”°ĻņÓŅ”±»ņ”°²»”±)ŅĘ¶Æ£»“ĖŹ±ČÜŅŗÖŠ ________(Ģī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±)”£
________(Ģī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±)”£
£Ø2£©ČōĻņ°±Ė®ÖŠ¼ÓČėµČÅضČĻ”“×Ėį£¬Ź¹ĘäĒ”ŗĆÖŠŗĶ£¬Š“³ö·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ_________________£»ĖłµĆČÜŅŗµÄpH________7(Ģī”°£¾”±”¢”°<”±»ņ”°£½”±)£¬
£Ø3£©ČōĻņ°±Ė®ÖŠ¼ÓČėĻ”ĮņĖįÖĮČÜŅŗµÄpH£½7£¬“ĖŹ±[NH4+]£½a mol”¤L£1£¬Ōņc(SO42-)£½________”£
£Ø4£©ČōĻņ°±Ė®ÖŠ¼ÓČėpH£½1µÄĮņĖį£¬ĒŅ°±Ė®ÓėĮņĖįµÄĢå»ż±ČĪŖ1”Ć1£¬ŌņĖłµĆČÜŅŗÖŠø÷Ąė×ÓµÄĪļÖŹµÄĮæÅضČÓɓ󵽊”µÄĖ³ŠņŹĒ________________________________________”£
£Ø1£©Ļņ×ó ¼õŠ” £Ø2£©NH3”¤H2O£«CH3COOH£½CH3COO££«NH4£«£«H2O £½ £Ø3£©

£Ø4£©c(NH4£«)£¾c(SO42£)£¾c(H£«)£¾c(OH£)
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©Ļņ°±Ė®ÖŠ¼ÓČėÉŁĮæĮņĖįļ§¹ĢĢ壬ĮņĖįļ§µēĄė³öĄ“µÄļ§øłĄė×Ó»įŅÖÖĘŅ»Ė®ŗĻ°±µÄµēĄė£¬Ę½ŗāĻņ×óŅĘ¶Æ£¬“ĖŹ±ČÜŅŗÖŠ ¼õŠ” £Ø2£©NH3”¤H2O£«CH3COOH£½CH3COO££«NH4£«£«H2O £»ŅņĪŖĒ”ŗĆÖŠŗĶ£¬ČÜŅŗĻŌÖŠŠŌ£¬ĖłµĆČÜŅŗµÄpH£½7 £Ø3£©ŅĄ¾ŻµēŗÉŹŲŗćÓŠ£ŗc(NH4£«)£«c(H£«)£½2c(SO42£)£«c(OH£)£¬ÓÖŅņĪŖČÜŅŗµÄpH£½7£¬Ņņ“ĖÓŠ£ŗc(SO42£)£½
¼õŠ” £Ø2£©NH3”¤H2O£«CH3COOH£½CH3COO££«NH4£«£«H2O £»ŅņĪŖĒ”ŗĆÖŠŗĶ£¬ČÜŅŗĻŌÖŠŠŌ£¬ĖłµĆČÜŅŗµÄpH£½7 £Ø3£©ŅĄ¾ŻµēŗÉŹŲŗćÓŠ£ŗc(NH4£«)£«c(H£«)£½2c(SO42£)£«c(OH£)£¬ÓÖŅņĪŖČÜŅŗµÄpH£½7£¬Ņņ“ĖÓŠ£ŗc(SO42£)£½ c(NH4£«)£½
c(NH4£«)£½ £»£Ø4£©ČōĻņ°±Ė®ÖŠ¼ÓČėpH£½1µÄĮņĖį£¬ĒŅ°±Ė®ÓėĮņĖįµÄĢå»ż±ČĪŖ1”Ć1£¬ŌņµĆµ½µÄŹĒĮņĖįļ§µÄČÜŅŗ£¬ŅņĪŖ°±øłĄė×ÓµÄĖ®½ā£¬Ź¹µĆČÜŅŗĻŌĖįŠŌ£¬Ņņ“ĖÓŠČēĻĀ¹ŲĻµ£ŗc(NH4£«)£¾c(SO42£)£¾c(H£«)£¾c(OH£)
£»£Ø4£©ČōĻņ°±Ė®ÖŠ¼ÓČėpH£½1µÄĮņĖį£¬ĒŅ°±Ė®ÓėĮņĖįµÄĢå»ż±ČĪŖ1”Ć1£¬ŌņµĆµ½µÄŹĒĮņĖįļ§µÄČÜŅŗ£¬ŅņĪŖ°±øłĄė×ÓµÄĖ®½ā£¬Ź¹µĆČÜŅŗĻŌĖįŠŌ£¬Ņņ“ĖÓŠČēĻĀ¹ŲĻµ£ŗc(NH4£«)£¾c(SO42£)£¾c(H£«)£¾c(OH£)
æ¼µć£ŗæ¼²éČõµē½āÖŹµÄ·“Ó¦”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĮņĖįŹĒĒæĖį£¬ÖŠŃ§½×¶Ī½«ĮņĖįŌŚĖ®ČÜŅŗÖŠæ“×öĶźČ«µēĄė”£µ«ŹĀŹµŹĒ£¬ĮņĖįŌŚĖ®ÖŠµÄµŚŅ»²½µēĄėŹĒĶźČ«µÄ£¬µŚ¶ž²½µēĄė²¢²»ĶźČ«£¬ĘäµēĄėĒéæöĪŖH2SO4=H£«£«HSO4-£¬HSO4- H£«£«SO42-”£
H£«£«SO42-”£
Ēė»Ų“šĻĀĮŠÓŠ¹ŲĪŹĢā£ŗ
£Ø1£©Na2SO4ČÜŅŗ³Ź________(Ģī”°ČõĖįŠŌ”±”¢”°ÖŠŠŌ”±»ņ”°Čõ¼īŠŌ”±)£¬ĘäĄķÓÉŹĒ__________________________________________”£(ÓĆĄė×Ó·½³ĢŹ½±ķŹ¾)”£
£Ø2£©H2SO4ČÜŅŗÓėBaCl2ČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ_________________________”£
£Ø3£©ŌŚ0.10 mol”¤L£1µÄNa2SO4ČÜŅŗÖŠ£¬ĻĀĮŠĄė×ÓÅØ¶ČµÄ¹ŲĻµÕżČ·µÄŹĒ________(ĢīŠ“±ąŗÅ)”£
| A£®c(Na£«)£½c(SO42-)£«c(HSO4-)£«c(H2SO4) |
| B£®c(OH£)£½c(HSO4-)£«c(H£«) |
| C£®c(Na£«)£«c(H£«)£½c(OH£)£«c(HSO4-)£«2c(SO42-) |
| D£®c(Na£«)£½2c(SO42-)£«2c(HSO4-) |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĪŖĮĖÖ¤Ć÷“×ĖįŹĒČõµē½āÖŹ£¬¼×”¢ŅŅ”¢±ū”¢¶””¢Īģ”¢¼ŗ”¢øżĘßČĖ·Ö±šŃ”ÓĆĻĀĮŠŹŌ¼Į½ųŠŠŹµŃé£ŗ
0.10 mol/L“×ĖįČÜŅŗ”¢0.10 mol/L“×ĖįÄĘČÜŅŗ”¢pH=3µÄŃĪĖį”¢pH=3µÄ“×Ėį”¢“×ĖįÄĘ¾§Ģ唢ĀČ»ÆÄĘ¾§Ģ唢¼×»ł³Č”¢pHŹŌÖ½”¢ÕōĮóĖ®”£
(1)¼×ÓĆpHŹŌÖ½²ā³ö0.10 mol/LµÄ“×ĖįČÜŅŗpH=4£¬ŌņČĻ¶Ø“×ĖįŹĒČõµē½āÖŹ£¬ÄćČĻĪŖÕāŅ»·½·ØÕżČ·Āš£æ ”£(Ģī”°ÕżČ·”±»ņ”°²»ÕżČ·”±)
(2)ŅŅČ”³ö10 mL 0.10 mol/LµÄ“×ĖįČÜŅŗ£¬ÓĆpHŹŌÖ½²ā³öĘäpH=a£¬Č»ŗóÓĆÕōĮóĖ®Ļ”ŹĶµ½1 000 mL£¬ŌŁÓĆpHŹŌÖ½²ā¶ØĘäpH=b£¬ŅŖČ·¶Ø“×ĖįŹĒČõµē½āÖŹ£¬Ōņa”¢bÓ¦øĆĀś×ćµÄ¹ŲĻµŹĒ (ÓƵȏ½»ņ²»µČŹ½±ķŹ¾)”£
(3)±ūČ”³ö10 mL 0.10 mol/L“×ĖįČÜŅŗ£¬µĪČė¼×»ł³ČŹŌŅŗ£¬ĻŌŗģÉ«£¬ŌŁ¼ÓČė“×ĖįÄĘ¾§Ģ壬ŃÕÉ«±ä³ČÉ«£¬ÄćČĻĪŖÕāŅ»·½·ØÄÜ·ńÖ¤Ć÷“×ĖįŹĒČõµē½āÖŹ£æ ”£(Ģī”°ÄÜ”±»ņ”°²»ÄÜ”±)
(4)¶”ÓĆpHŹŌÖ½Ą“²ā¶Ø0.10 mol/L“×ĖįÄĘČÜŅŗµÄpH£¬·¢ĻÖ0.10 mol/L“×ĖįÄĘČÜŅŗµÄpHĪŖ9£¬ŌņČĻ¶Ø“×ĖįŹĒČõµē½āÖŹ£¬ÄćČĻĪŖÕāŅ»·½·ØÕżČ·Āš£æ ”£(Ģī”°ÕżČ·”±»ņ”°²»ÕżČ·”±)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
X”¢Y”¢Z”¢W·Ö±šŹĒHNO3”¢NH4NO3”¢NaOH”¢NaNO2ĖÄÖÖĒæµē½āÖŹÖŠµÄŅ»ÖÖ”£ĻĀ±ķŹĒ³£ĪĀĻĀÅØ¶Č¾łĪŖ0.0 1 mol”¤L-1µÄX”¢Y”¢Z”¢WČÜŅŗµÄpH”£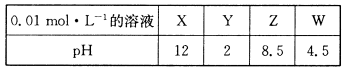
£Ø1£©X”¢WµÄ»ÆѧŹ½·Ö±šĪŖ_______”¢________”£
£Ø2£©WµÄµēĄė·½³ĢŹ½ĪŖ______________________________”£
£Ø3£©25”ꏱ£¬ZČÜŅŗµÄpH£¾7µÄŌŅņŹĒ______________________________________(ÓĆĄė×Ó·½³ĢŹ½±ķŹ¾)”£
£Ø4£©½«X”¢Y”¢Zø÷1mol”¤L-1Ķ¬Ź±ČÜÓŚĖ®ÖŠÖʵƻģŗĻČÜŅŗ£¬Ōņ»ģŗĻČÜŅŗÖŠø÷Ąė×ÓµÄÅضČÓɓ󵽊”µÄĖ³ŠņĪŖ _______________________________________________________ ”£
£Ø5£©ZČÜŅŗÓėWČÜŅŗ»ģŗĻ¼ÓČČ£¬æɲśÉśŅ»ÖÖĪŽÉ«ĪŽĪ¶µÄµ„ÖŹĘųĢ壬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ__________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ä³Ń§ÉśÓūÓĆŅŃÖŖĪļÖŹµÄĮæÅØ¶ČµÄ“×ĖįĄ“²ā¶ØĪ“ÖŖĪļÖŹµÄĮæÅØ¶ČµÄĒāŃõ»ÆÄĘČÜŅŗŹ±£¬Ń”ŌńŹŹµ±µÄÖøŹ¾¼Į”£ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ
£Ø1£©ÓƱź×¼“×ĖįµĪ¶Ø“ż²āµÄĒāŃõ»ÆÄĘČÜŅŗŹ±£¬“ÓĻĀĮŠŃ”ĻīÖŠŃ”³ö×īĒ”µ±µÄŅ»Ļī________”£
| | ׶ŠĪĘæÖŠČÜŅŗ | µĪ¶Ø¹ÜÖŠČÜŅŗ | Ń”ÓĆÖøŹ¾¼Į | Ń”ÓƵĪ¶Ø¹Ü |
| A | ¼ī | Ėį | ŹÆČļ | £ØŅŅ£© |
| B | Ėį | ¼ī | ¼×»ł³Č | £Ø¼×£© |
| C | ¼ī | Ėį | ·ÓĢŖ | £Ø¼×£© |
| D | Ėį | ¼ī | ŹÆČļ | £ØŅŅ£© |
| | “ż²āĒāŃõ»ÆÄĘ | 0.100mol/L“×ĖįµÄĢå»ż | |
| µĪ¶Ø“ĪŹż | ČÜŅŗµÄĢå»ż£Øml£© | µĪ¶ØĒ°µÄæĢ¶Č£Øml£© | µĪ¶ØŗóµÄæĢ¶Č£Øml£© |
| µŚŅ»“Ī | 25.00 | 0.00 | 24.98 |
| µŚ¶ž“Ī | 25.00 | 1.56 | 27.86 |
| µŚČż“Ī | 25.00 | 0.22 | 25.24 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
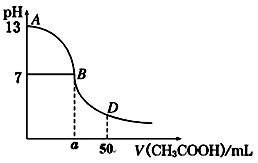
£Ø1£©ŅŃÖŖ³£ĪĀĻĀ£¬AĖįµÄČÜŅŗpH=a£¬B¼īµÄČÜŅŗpH=b£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
¢ŁČōAĪŖ“×Ėį£¬BĪŖĒāŃõ»ÆÄĘ£¬ĒŅa=4£¬b=10£¬Į½ÕßµČĢå»ż»ģŗĻ£¬Ōņ»ģŗĻĒ°c(CH3COOH) c(NaOH )£ØĢī”°£¼”¢£½»ņ£¾”±£¬ĻĀĶ¬£©£»»ģŗĻŗóČÜŅŗµÄpH 7”£
¢ŚČōAµÄ»ÆѧŹ½ĪŖHR£¬BµÄ»ÆѧŹ½ĪŖMOH£¬ĒŅa+b=14£¬Į½ÕßµČĢå»ż»ģŗĻŗóČÜŅŗĻŌ¼īŠŌ£¬Ōņ»ģŗĻČÜŅŗÖŠ±Ų¶ØÓŠŅ»ÖÖĄė×ÓÄÜ·¢ÉśĖ®½ā£¬øĆĖ®½ā·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”££Ø2£©¢ŁĻÖÓŠ25”ꏱµČĢå»ż”¢µČpHµÄBa(OH)2”¢NaOHŗĶNH3”¤H2OČżÖÖČÜŅŗ£¬½«ĖüĆĒ·Ö±šÓėV1 L”¢V2 L”¢V3 LµČÅØ¶ČµÄŃĪĖį»ģŗĻ£¬¾łĒ”ŗĆÖŠŗĶ£¬ŌņV1”¢V2”¢V3µÄ“󊔹ŲĻµŹĒ £»
¢ŚĮķÓŠ25”ę£¬µČĢå»żµČĪļÖŹµÄĮæÅØ¶ČµÄBa(OH)2”¢NaOHŗĶNH3”¤H2OČżÖÖČÜŅŗ£¬½«ĖüĆĒ·Ö±šÓėV1 L”¢V2 L”¢V3 LµČÅØ¶ČµÄŃĪĖį»ģŗĻ£¬»ģŗĻŗóČÜŅŗ¾ł³ŹÖŠŠŌ£¬ŌņV1”¢V2”¢V3µÄ“󊔹ŲĻµŹĒ £»¢ŪŹŅĪĀĻĀ£¬ČōÉś³ÉĶ¬ÅØ¶ČµÄNaClÓėNH4ClČÜŅŗø÷1L£¬ŌņČÜŅŗÖŠĄė×Ó×ÜŹżN(NaCl) N(NH4Cl)”££ØĢī”°£¼”¢£½»ņ£¾”±£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø1£©ŹŅĪĀĻĀŌŚpH=12µÄNaCNČÜŅŗÖŠ£¬ÓÉĖ®µēĄėµÄc(OH”Ŗ)ĪŖ mol?L”Ŗ1”£
£Ø2£©ÅضČĪŖ0.1mol?L”Ŗ1µÄĻĀĮŠø÷ĪļÖŹµÄČÜŅŗÖŠ£¬c(NH4+)Óɓ󵽊”µÄĖ³ŠņŹĒ___£ØĢīŠņŗÅ£©”£
¢ŁNH4Cl ¢ŚNH4HSO4 ¢ŪNH3?H2O ¢ÜCH3COONH4
£Ø3£©Ä³¶žŌŖĖį£Ø»ÆѧŹ½ÓĆH2A±ķŹ¾£©ŌŚĖ®ÖŠµÄµēĄė·½³ĢŹ½ŹĒ£ŗ
H2A=H+ +HA”Ŗ£¬HA”„ H+ +A2”Ŗ”£
H+ +A2”Ŗ”£
¢ŁŌņNa2AČÜŅŗĻŌ____ŠŌ£»NaHAČÜŅŗĻŌ ŠŌ£ØĢī”°ĖįŠŌ”±”¢”°ÖŠŠŌ”±»ņ”°¼īŠŌ”±£©”£
¢ŚČōÓŠ0.1mo1?L”Ŗ1Na2AµÄČÜŅŗ£¬ĘäÖŠø÷ÖÖĄė×ÓÅضČÓɓ󵽊”µÄĖ³ŠņŹĒ£ŗ £ØĢīŠņŗÅ£©”£
| A£®c(Na+)>c(A2”Ŗ)>c(OH”Ŗ)>c(HA”Ŗ)>c(H+) |
| B£®c(Na+)> c(OH”Ŗ)>c(HA”Ŗ)> >c(A2”Ŗ) > c(H+) |
| C£®c(Na+)> c(H+)> c(A2”Ŗ)> c(OH”Ŗ)>c(HA”Ŗ) |
| D£®c(A2”Ŗ)>c(Na+)> c(OH”Ŗ) > c(H+)>c(HA”Ŗ) |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĻÖÓŠpH£½2µÄ“×ĖįČÜŅŗ¼×ŗĶpH£½2µÄŃĪĖįŅŅ£¬Ēėøł¾ŻĻĀĮŠ²Ł×÷Ķź³ÉĪŹĢā£ŗ
(1)Č”10 mLµÄ¼×ČÜŅŗ£¬¼ÓČėµČĢå»żµÄĖ®£¬“×ĖįµÄµēĄėĘ½ŗā________(Ģī”°Ļņ×ó”±”¢”°ĻņÓŅ”±»ņ”°²»”±)ŅĘ¶Æ£»ĮķČ”10 mLµÄ¼×ČÜŅŗ£¬¼ÓČėÉŁĮæĪŽĖ®“×ĖįÄĘ¹ĢĢå(¼ŁÉč¼ÓČė¹ĢĢåĒ°ŗó£¬ČÜŅŗĢå»ż±£³Ö²»±ä)£¬“ż¹ĢĢåČÜĶźŗó£¬ČÜŅŗÖŠ[H£«]/[CH3COOH]µÄ±ČÖµ½«________(Ģī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°ĪŽ·ØČ·¶Ø”±)”£
(2)ĻąĶ¬Ģõ¼žĻĀ£¬Č”µČĢå»żµÄ¼×”¢ŅŅĮ½ČÜŅŗ£¬ø÷Ļ”ŹĶ100±¶”£Ļ”ŹĶŗóµÄČÜŅŗ£¬ĘäpH“󊔹ŲĻµĪŖ£ŗpH(¼×)________pH(ŅŅ)”£(Ģī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±£¬ĻĀĶ¬)
(3)ø÷Č”25 mLµÄ¼×”¢ŅŅĮ½ČÜŅŗ£¬·Ö±šÓƵČÅØ¶ČµÄNaOHĻ”ČÜŅŗÖŠŗĶÖĮpH£½7£¬ŌņĻūŗĵÄNaOHČÜŅŗµÄĢå»ż“󊔹ŲĻµĪŖ£ŗV(¼×)________V(ŅŅ)”£
(4)Č”25 mLµÄ¼×ČÜŅŗ£¬¼ÓČėµČĢå»żpH£½12µÄNaOHČÜŅŗ£¬·“Ó¦ŗóČÜŅŗÖŠ[Na£«]”¢[CH3COO£]µÄ“󊔹ŲĻµĪŖ£ŗ[Na£«]________[CH3COO£]”£
(5)Č”25 mLµÄŅŅČÜŅŗ£¬¼ÓČėµČĢå»żpH£½12µÄ°±Ė®£¬·“Ó¦ŗóČÜŅŗÖŠµÄČÜÖŹĪŖ_________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ä³Ń§Ļ°Š”×éÓūĢ½¾æCaSO4³Įµķ×Ŗ»ÆĪŖCaCO3³Įµķ£¬“Ó¶ų½«Ęä³żČ„µÄæÉÄÜŠŌ£¬²éµĆČēĻĀ׏ĮĻ£ŗ(ŅŌĻĀŹż¾ŻŗĶŹµŃé¾łÖøŌŚ25”ęĻĀ²ā¶Ø)
| ÄŃČܵē½āÖŹ | CaCO3 | CaSO4 |
| Ksp(mol2”¤L£2) | 3”Į10£9 | 9”Į10£6 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com