
ij��ѧС���������������������װ�ã�����ͼ�����Ի������Ʊ�����ϩ


| | �ܶȣ�g/cm3�� | �۵㣨�棩 | �е㣨�棩 | �ܽ��� |
| ������ | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | -103 | 83 | ������ˮ |

��1���� �����У����� �ڷ�ֹ����ϩ�ӷ�����������ϩ
��2���� �ϣ� CD �� g����ˮ �� 830C��C��3��B
���������������1���ٸ�������ϩʵ���֪ʶ������װ��A�����Ƭ�������Ƿ�ֹ���У��������ɵĻ���ϩ�ķе�Ϊ83�棬Ҫ�õ�Һ̬����ϩ������B���˵���������������ã����ڻ���ϩ������
�ڱ�ˮԡ��Ŀ���ǽ��ͻ���ϩ�������¶ȣ�ʹ��Һ����
��2���ٻ���ϩ�������Ȼ�����Һ�����ܶȱ�ˮС���ֲ��ϩ���ϲ㣬���ڷ�Һ��ϩ��Ʒ�л�������������ͻ��������ᴿ����ʱ��c��Na2CO3��Һ��ϴ�ӿɳ�ȥ�
��Ϊ����������Ч������ȴˮ���¿ڣ�g�����룬��ȴˮ�������γ�����������Ч�����ã������������ܳ���ˮ���Է�������ڴ���������ʹ���������ѣ�
�۸��ݱ������ݿ�֪����ֻ���ϩ�ķе�Ϊ83�棻
a������ǰ�ռ�����Ʒ�л������ʣ�ʵ�ʲ����������۲�����
b����ȡ�Ļ���ϩ���ʵ�������ʵ���ƵõĻ���ϩ��Ʒ�����������۲�����
c���ֲ�Ʒ�л��л����������²ⶨ���ĵĻ������������ƵõĻ���ϩ��Ʒ�����������۲�����
��3�����ݻ����û�й̶��ķе㣬���������й̶��ķе㣬�ݴ˿��жϲ�Ʒ�Ĵ��ȡ�
���㣺�����������������Ʊ�ʵ�顢Һ�������ʵ�������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
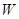 ��һ���л��ϳ��м��壬�ṹ��ʽΪ��HOOC��CH=CH��CH=CH��COOH��
��һ���л��ϳ��м��壬�ṹ��ʽΪ��HOOC��CH=CH��CH=CH��COOH��
��1��W�ܷ�����Ӧ�������� ������д��ĸ��ţ�
A��ȡ����Ӧ B��ˮ�ⷴӦ C��������Ӧ D���ӳɷ�Ӧ
��2����֪ Ϊƽ����ƽ��ṹ����W����������� ��ԭ����ͬһƽ���ڡ�
Ϊƽ����ƽ��ṹ����W����������� ��ԭ����ͬһƽ���ڡ�
��3��������Q�� ����һ��ҩ���м��壬���ںϳɿ���ҩ������˳����Q��W��ϵ����������ȷ����
����һ��ҩ���м��壬���ںϳɿ���ҩ������˳����Q��W��ϵ����������ȷ����
A���������������� B�����Dz����Ͷ�Ԫ����
C����Ϊͬϵ�� D����Ϊͬ���칹��
��4��W�ϳɷ������£�
��֪����CHO + (C6H5)3P=CH��R����CH=CH��R + (C6H5)3P=O��R����ԭ�ӻ�ԭ���š�
���У� �ֱ����һ���л���ϳɹ�������������ͷ�Ӧ��������ȥ��
�ֱ����һ���л���ϳɹ�������������ͷ�Ӧ��������ȥ��
X��W��һ�������·�Ӧ����������N��N����Է�������Ϊ168��д��X�� W��һ�������·�Ӧ����N�Ļ�ѧ����ʽ�� ��
��5��д���ڢڲ���Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��6�֣���11. 2L��״���µļ������ϩ�Ļ������ͨ��������������Ȼ�̼��Һ�г�ַ�Ӧ��������Ȼ�̼��Һ������5.6g����ԭ��������м�������ϩ������֮�ȡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��6�֣�ij��ѧ��ȤС���üס�������װ�ã�����ͼ��ʾ�����м��ᣨHCOOH����״���CH318OH��������Ӧ��ʵ�飬�ش��������⣺ 
��1�����ᣨHCOOH���ͼ״���CH318OH������������Ӧ�Ļ�ѧ����ʽ�ǣ�
___ _��
��2����װ���г���������c��������__ ___��
��3���ס�������װ����Ч���ȽϺõ�װ���� ��ԭ��
��4���Թ�A����ƿB��װ���� ������Ϊ
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ʵ�����Ʊ����������Ļ�ѧ����ʽ��CH3COOH��C2H5OH CH3COOC2H5��H2O��Ϊ֤��Ũ�����ڸ÷�Ӧ�����˴��������ռ������ã�ijͬѧ������ͼ��ʾװ�ý����������ĸ�ʵ�飬ʵ�鿪ʼ���þƾ�����3 min���ټ���ʹ֮����3 min��ʵ������������Թܢ��ٲ��л���ĺ�ȣ�ʵ���¼���£�
CH3COOC2H5��H2O��Ϊ֤��Ũ�����ڸ÷�Ӧ�����˴��������ռ������ã�ijͬѧ������ͼ��ʾװ�ý����������ĸ�ʵ�飬ʵ�鿪ʼ���þƾ�����3 min���ټ���ʹ֮����3 min��ʵ������������Թܢ��ٲ��л���ĺ�ȣ�ʵ���¼���£�
| ʵ���� | �Թܢ��е��Լ� | �Թܢ����Լ� | ����л���ĺ��/cm |
| A | 2 mL�Ҵ���2 mL���ᡢ1 mL 18 mol��L��1Ũ���� | ����̼������Һ | 5.0 |
| B | 3 mL�Ҵ���2 mL���� | 0.1 | |
| C | 3 mL�Ҵ���2 mL���ᡢ6mL 3 mol��L��1���� | 1.2 | |
| D | 3 mL�Ҵ���2 mL���ᡢ���� | 1.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�ⶨ�л�����̼�����Ԫ�صĺ�������õķ�����ȼ�շ�������������֪������Ʒ�����������У�������ͭ����������760�����ң���Ʒȫ��������Ϊ������̼��ˮ��ʵ��װ������ͼ��ʾ��
��1��ʵ��ʱ��װ��a��b��ͨ��װ������ʷֱ��� �� ��
��2����ʼ����֮ǰ��Ҫͨһ��ʱ���������Ŀ���� ��ֹͣ���Ⱥ�ҲҪͨһ��ʱ���������Ŀ���� ��
��3��ij��ʵ���У�ȡ����Ʒ���ĺ���������A������Ϊ2.3g�����ⶨaװ������2.7g��bװ������4.4g���������A���ʵ�ʵ��ʽ: ��Ҫ��д��������̣�
��4������һ���ⶨ��֪A����Է�������Ϊ46����A�ķ���ʽΪ ��
��5��ʵ�鷢��A�����������ƿ��Էų���������A�����о��еĹ�����Ϊ ��ͬ��ȡ2.3g��A�����������Ʒ�Ӧ������ͼ��ʾװ����ȡ�ų�����������ȡ����ʱ����ˮ�ܵ�ע������Ϊ ������ȡ�����廻��ɱ�״�����ΪcmL����һ��A�������� ����ԭ�ӱ��û��ˣ��ú�c�ı���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����ˮ�����׳ư�˾ƥ�֣���һ����ʷ�ƾõĽ�����ʹҩ���ϳ�ԭ���ǣ�
�����Ϊ�ᴿԭ������Ϊ�˳�ȥ�÷�Ӧ�ĸ����ˮ������������ˮ����ˮ����������ˮ�������;ۺ���ȡ�ˮ���ᣨ�۵�158�棩������ˮ���ᣨ�۵�135�棩������ˮ����������ˮ���������������������ǣ�
��1������I��III�������� ��ʹ����ˮԡ���ȵ�ԭ���� ��
��2������ѭ�����õ��� ������2�ijɷ��� ��
��3��Ϊ�˼����Ʒ���Ƿ���ˮ���ᣬ�����о��ƣ���ȥ��Ʒ�е�������Ӧ���������������²�����������±���
| ��� | ���� | ���� | ���� |
| �� �� | ȡ������Ʒ����Ͷ��װ������ˮ���Թ��У��� | ����ɫ��Һ | |
| ���Թ��е��� ��Һ | . | ��Ʒ����ˮ���� | |
| �� �ᾧ | ���ֲ�Ʒ����������ˮ�У�ˮԡ���ȣ����ȹ��ˣ�����Һ ������ | �о������� | ����Ʒ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���й��ڼ�����ӽṹ�������У���ȷ����
A�����������C��Hԭ�Ӽ�Ϊ���Ӽ�
B��������ӵĿռ�ṹ��������
C������ĽṹʽΪCH4
D�����������4��̼�����ȫ��ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
ͨʽΪCnH2n-2��һ����̬����ȫȼ�պ�����CO2��H2O�����ʵ���֮��Ϊ4��3������������״ͬ���칹���У� ��
| A��2�� | B��3�� | C��4�� | D��5�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com