���㣺������ƶ�,���ӷ���ʽ���йؼ���
ר�⣺�ƶ���,������
��������1����2����3����������ɫ������Na
2O
2��S������B����ˮ��Ӧ������������BΪNa
2O
2��AΪNa��CΪNaOH��D����������������Ӧ����DΪS��EΪSO
2��FΪSO
3��GΪH
2SO
4��G��NaOH�����кͷ�Ӧ����HΪNa
2SO
4��
��4����Al���������Ʒ�Ӧ����ƫ�����ơ�������ˮ��
��n��H
2SO
4��=1.5mol/L��0.1L=0.15mol��n��NaOH��=3mol/L��0.1L=0.3mol��n��Al��=
=0.1mol������2Al+3H
2SO
4=Al
2��SO
4��
2+3H
2����Al��H
2SO
4��ȫ��Ӧ��Ҫn��H
2SO
4��=
n��Al��=
��0.1mol=0.15mol������Al��H
2SO
4ǡ����ȫ��Ӧ����Al
2��SO
4��
2���ټ���NaOH��Һ�������õ���������������
����2Al+2NaOH+2H
2O=2NaAlO
2+3H
2����Al��NaOH��ȫ��Ӧ��Ҫn��NaOH��=n��Al��=0.1mol��0.3mol������NaOH��ʣ�࣬�ټ������ᣬ�ȷ����кͷ�ӦH
++OH
-=H
2O���ٷ�����H
++AlO
2-+H
2O=Al��OH��
3����
�۸���������غ����n��H
2SO
4������Һ���Ϊ����������������Һ���֮�ͣ������������������ʵ���Ũ�ȣ�
���
�⣺��������ɫ������Na
2O
2��S������B����ˮ��Ӧ������������BΪNa
2O
2��AΪNa��CΪNaOH��D����������������Ӧ����DΪS��EΪSO
2��FΪSO
3��GΪH
2SO
4��G��NaOH�����кͷ�Ӧ����HΪNa
2SO
4��
��1�������Ϸ�����֪��BΪNa
2O
2���ʴ�Ϊ��Na
2O
2��
��2��B��C�ķ�ӦΪ2Na
2O
2+2H
2O=4NaOH+O
2�����ʴ�Ϊ��2Na
2O
2+2H
2O=4NaOH+O
2����
��3��EΪSO
2������OH
-��ClO
-��NO
3-�����ԣ������������ܴ����������C���ʴ�Ϊ��C��
��4��������NaOH��Һ��Ӧ�����ӷ���ʽΪ��2Al+2OH
-+2H
2O=2AlO
2-+3H
2�����ʴ�Ϊ��2Al+2OH
-+2H
2O=2AlO
2-+3H
2����
��n��H
2SO
4��=1.5mol/L��0.1L=0.15mol��n��NaOH��=3mol/L��0.1L=0.3mol��n��Al��=
=0.1mol������2Al+3H
2SO
4=Al
2��SO
4��
2+3H
2����Al��H
2SO
4��ȫ��Ӧ��Ҫn��H
2SO
4��=
n��Al��=
��0.1mol=0.15mol������Al��H
2SO
4ǡ����ȫ��Ӧ����Al
2��SO
4��
2���ټ���NaOH��Һ�������õ���������������
����2Al+2NaOH+2H
2O=2NaAlO
2+3H
2����Al��NaOH��ȫ��Ӧ��Ҫn��NaOH��=n��Al��=0.1mol��0.3mol������NaOH��ʣ�࣬�ټ������ᣬ�ȷ����кͷ�ӦH
++OH
-=H
2O���ٷ�����H
++AlO
2-+H
2O=Al��OH��
3����
��Ob�η�����Ӧ�����ӷ���ʽΪ��H
++AlO
2-+H
2O=Al��OH��
3����
�ʴ�Ϊ��H
++AlO
2-+H
2O=Al��OH��
3����
��O��ʱ��������������Һ���ʾ�ΪNa
2SO
4����Һ���Ϊ����������������Һ���֮�ͣ�����������غ�n��Na
2SO
4��=n��H
2SO
4��=0.15mol����c��Na
2SO
4��=
=0.75mol/mol��
�ʴ�Ϊ��0.75mol/L��
���������⿼��������ƶϡ�����ʵ�鷽����ƣ���4���йؼ��Ǹ��ݹ���������ȷ���߶η����Ļ�ѧ��Ӧ�����ؿ���ѧ���������⡢�������������ע����ػ���֪ʶ�Ļ��ۣ�





 �»����ܶ�Ա��ϵ�д�
�»����ܶ�Ա��ϵ�д� ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
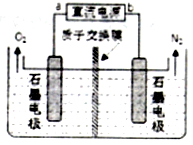
 �Է�����ͼ��ʾ�ķ��ӽṹ���ش��������⣺
�Է�����ͼ��ʾ�ķ��ӽṹ���ش��������⣺