
(12��)����ѧ������ѧ�뼼����
��Դ�����������Ʊ�����ũҵ�����ȶ��벻����ѧ����ش��������⣺
��1����ҵ�Ʒ���ʱ����������Ӧ��������Ҫ�ڻ�����м��뱥��ʳ��ˮ�����뱥��ʳ��ˮ��Ŀ���� ��
��2��Al2O3���۵�ߴ�2050oC����ҵ��Ϊ�˽����������ģ��ڽ�������ұ����ͨ����ȡ�Ĵ�ʩ�� ��
��3����ҵ�Ϻϳɰ�����ĵ�����Դ�� ��������Դ�� ��д����ҵ���ڴ�����������ȡ����������һ����ѧ����ʽ ��
��4����ҵ������ʱ��SO3���������� (���豸����)�н��еģ���ҵ�ϳ�����Ũ��������SO3������ֱ����ˮ���յ�ԭ���� �����������У�Ϊ���SO3������������ȡ�Ĵ�ʩΪ ��
��1��������ʹ�����ӻ��Һ�з����������2�����������м������ʯ�����ͻ������۵㡣��3��������ˮ��̼�⻯���CH4+H2O CO+H2��CH4+2H2O
CO+H2��CH4+2H2O CO2+2H2����4���Ӵ��ң���ֹ�γ�������Ӱ��SO3������Ч�ʡ��������²�ͨ��SO3�������ϲ�����ŨH2SO4��SO3��ŨH2SO4�����ϱ���Ӵ��������ա�
CO2+2H2����4���Ӵ��ң���ֹ�γ�������Ӱ��SO3������Ч�ʡ��������²�ͨ��SO3�������ϲ�����ŨH2SO4��SO3��ŨH2SO4�����ϱ���Ӵ��������ա�
������������� ��1����ҵ�Ʒ���ʱ�����뱥��ʳ��ˮ��Ŀ����������ʹ�����ӻ��Һ�з����������2��Al2O3���۵�ߴ�2050oC����ҵ��Ϊ�˽����������ģ��ڽ�������ұ����ͨ����ȡ�Ĵ�ʩ�����������м������ʯ�����ͻ������۵㡣��3����ҵ�Ϻϳɰ�����ĵ�����Դ�ڿ�����������Դ��ˮ��̼�⻯�����ҵ���ڴ�����������ȡ�����Ļ�ѧ����ʽΪCH4+H2O CO+H2��CH4+2H2O
CO+H2��CH4+2H2O CO2+2H2����4����ҵ������ʱ��SO3���������ڽӴ����н��еģ���ҵ�ϳ�����Ũ��������SO3������ֱ����ˮ���յ�ԭ���Ƿ�ֹ�γ�������Ӱ��SO3������Ч�ʡ����������У�Ϊ���SO3������������ȡ�Ĵ�ʩΪ�������²�ͨ��SO3�������ϲ�����ŨH2SO4��SO3��ŨH2SO4�����ϱ���Ӵ��������ա�
CO2+2H2����4����ҵ������ʱ��SO3���������ڽӴ����н��еģ���ҵ�ϳ�����Ũ��������SO3������ֱ����ˮ���յ�ԭ���Ƿ�ֹ�γ�������Ӱ��SO3������Ч�ʡ����������У�Ϊ���SO3������������ȡ�Ĵ�ʩΪ�������²�ͨ��SO3�������ϲ�����ŨH2SO4��SO3��ŨH2SO4�����ϱ���Ӵ��������ա�
���㣺���黯ѧ�뼼�����漰��������ȡ������ұ�����ϳɰ������Ṥҵ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
[��ѧ��ѡ��2��ѧ�뼼��](15��)
��1�����й��ڹ�ҵ����˵����ȷ���� ��������ţ�
| A���ں����Ƽҵ�У����Ȼ�����Һ����ͨ������̼����ͨ���� |
| B�������Ṥҵ���ϳɰ���ҵ�����Ṥҵ�У��Բ���ѭ���������ԭ�������� |
| C�����ȼҵ�����۱����ӽ���Ĥ���������Һ������� |
| D����ҵ�ϲ��õ�������Ȼ����ķ�����ȡ������ |
| pH | Ca2+ ��Mg2+��Ũ�� | ϸ������ |
| 6.5��8.5 | �� 0.004 5 mol��L-1? | ��100����mL-1? |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ĺ�ˮ����Լ��1.4��1018t��������������Դ�⡣
��1����ͼ���ú�ˮ�õ���ˮ�ķ���Ϊ ��
��2�����������ǽ��귢չ������һ�ֽϺõĺ�ˮ������������ԭ������ͼ��a�ǵ�Դ�� ��������ų����� �����ˮ�� ��Ũˮ������
��3����ˮ�������Ũˮ�к������η֣���Ҫ����Mg2����Ca2+��Fe3����SO42����������ˮ�л�ı�ˮ�ʣ��ŵ������лᵼ�������μ���ʲ���ֱ���ŷţ��������ȼҵ������
���ǰ��Ҫ��Ũˮ���ƣ������Լ���Ҫ������HCl��NaOH��BaCl2��Na2CO3�ȣ�������HCl��������Ҫ�� ��
��4�����Ǻ˷�Ӧ����Ҫ��ȼ�ϣ�����������ֱ�ӹ�ϵ��һ�����Һ˹�ҵ��������ķ�չˮƽ����ˮ������UCl4��ʽ���ڣ���������ʽ���ڣ���ÿ�ֺ�ˮֻ��3.3�����ˣ���ˮ���������������൱���ٹ�������̽����ˮ���˵ķ��������ڣ��Ѿ����Ƴɹ�һ�����������ӽ�����֬����ר��������ˮ�е��ˣ�������������Ԫ�ء��䷴Ӧԭ��Ϊ��___________________________����֬��HR���棩���������ӽ���������ӽ���Ĥ���ᴦ�������������õ����˵���Һ���䷴Ӧԭ��Ϊ��________________________��
��5�����ӽ�����֬�����Ʊ���ˮ��ȥ����ˮ�� ����Ҫ������ij�����ӽ��������ľֲ��ṹ��д�ɣ���ͼ�����������ӽ�����֬���ɵ��屽��ϩ�ͽ������Զ���ϩ�����ۺϺ��پ� ��Ӧ�õ��ġ�����ˮ������ӽ�����֬�������ú��� ������ԡ��������ԡ������ԡ�����
��6���й���������ˮ���������й涨��ˮ����Ӳ�Ȳ��ܹ������Ӳ�ȹ������ú�����彡�����ճ�������һ��Ӱ�졣��ʱӲˮ��Ӳ������ ���������ӷ��ţ�����ģ��� ����������ƣ���ɱ�ȥ��������Ӳˮ��Ӳ�ȿ������ӽ�����ȥ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��.��ҵ������������ȡ�����������£�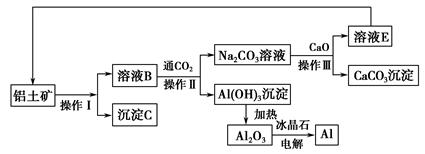
��ش��������⣺
(1)���������������õ��IJ���������__________________________��
(2)д������ҺB����Al(OH)3�����ӷ���ʽ��____________________________��
(3)�����������漰������ԭ��Ӧ�Ļ�ѧ����ʽΪ��_________________________��
(4)���������У���NaOH��H2O����ѭ��ʹ���⣬������ѭ��ʹ�õ�������________(�ѧʽ)���ô˷���ȡ���ĸ���Ʒ��________(�ѧʽ)��
��.�ҹ���¯����������ȡ��������������������Դ����Դ�����ʡ���¯���ͻ�����߲�ҵ���ж��Լ������ȷ��滹�����ʴ��ںܴ��࣬�д���һ����ߣ�Ŭ�������ǿ����������ش��������⣺
(1)��¯������ԭ��������̿��ʯ��ʯ���������ۼ����õ���________��Ŀ���dz�ȥ����ʯ�е���ʯ��������ܶȱ���________����������ˮ��________(��ϲ������²���)�γ�¯��������ˮ���롣
(2)��̿�ڸ�¯���������ž������ص����ã����в����ڽ�̿���õ���________��
A����Ϊȼ�ϣ�Ϊ�����еĻ�ѧ��Ӧ�ṩ����
B����Ϊ��ԭ���������̼��Ӧ������ԭ��������һ����̼
C���Ը�¯�е�������֧�ź���ɢ������
D����Ϊ�ܼ�����ȥ����ʯ�е�����
(3)��¯��������Ⱦ�dz����أ�Ŀǰ�ҹ����ִ�����еĸ��������Ű�Ǩ�Ļ���Ҳ�ڽ����Ź��ոĽ�����¯�������µĻ�����Ⱦ��________��
A�������ն� B������ C��һ����̼���� D��ɳ����
(4)д����¯��������̼Ԫ���йص�������ԭ��Ӧ��ѧ����ʽ��____________________________��
(5)�ӡ����ϡ����á����������ȽǶȿ��ǣ�������ҵ��������Ӧ��ȡ��һЩ��ʩ��(�ٳ�2�ִ�ʩ����) _______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ҹ��зḻ����Ȼ����Դ������Ȼ��Ϊԭ�Ϻϳ����ص���Ҫ������ͼ��ʾ(ͼ��ijЩת�����輰������δ�г�):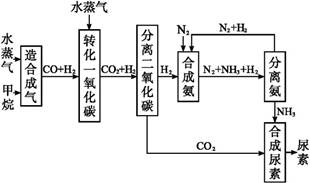
����д���пհ�:
(1)��֪0.5 mol�����0.5 molˮ������t�桢p kPaʱ,��ȫ��Ӧ����һ����̼������(�ϳ���),������a kJ�������÷�Ӧ���Ȼ�ѧ����ʽ����������������
(2)����������,��ҵ�Ϸ���H2��CO2�����ķ�������������
| A���������ͨ������������Һ,������Һ�м������� |
| B���������ѹ��ȴ,ʹCO2Һ�� |
| C��������ð�ˮϴ�� |
| D���������ͨ��ʯ�ҽ���,Ȼ��������չ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���Ų��Ͽ�ѧ�ķ�չ�����������仯����õ���Խ��Խ�㷺��Ӧ�ã�������Ϊ���Ͻ�ά���ء�����ҵ�ϻ��շϷ�����������V2O5��VOSO4��K2SO4��SiO2���з�����Ҫ�������£�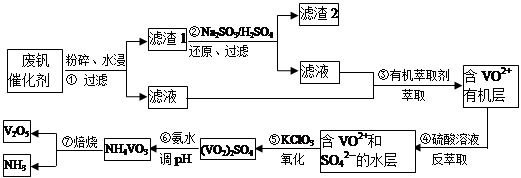
��֪����1��V2O5��NH4VO3��Ϊ�����VOSO4��(VO2)2SO4��Ϊ�����
��2�� 2VO2++H2C2O4+2H+ = 2VO2+ + 2CO2��+ 2H2O
�ش��������⣺
��1�������ǰ�������Ŀ����_________________________��
��2��������з�����Ӧ�����ӷ���ʽΪ__________________________��
��3������۵ı仯���̿ɼ�Ϊ(HA��ʾ�л���ȡ��)��
VOSO4 (ˮ��)+ 2HA���л��㣩 VOA2(�л��㣩+ H2SO4(ˮ��)��������п�ѡ������������ȡ��ԭ����_____________��
VOA2(�л��㣩+ H2SO4(ˮ��)��������п�ѡ������������ȡ��ԭ����_____________��
��4���������ữ��H2C2O4��Һ�ζ�(VO2)2SO4��Һ���Բⶨ�����ݺ���Һ�к������IJ���Ϊ��ȡ10.0mL0.1mol/LH2C2O4��Һ����ƿ�У�����ָ�����������Һʢ���ڵζ����У��ζ����յ�ʱ�����Ĵ���Һ�����Ϊ10.0mL���ɴ˿�֪(VO2)2SO4��Һ��Ԫ�صĺ���Ϊ_________g/L��
��5��V2O5���ý���(��Ca��Al)�Ȼ�ԭ����÷�����������Ȼ�ԭ�Ƶ÷��Ļ�ѧ����ʽΪ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������ƣ�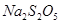 ��������ʳƷƯ�������Ʊ������������£�
��������ʳƷƯ�������Ʊ������������£�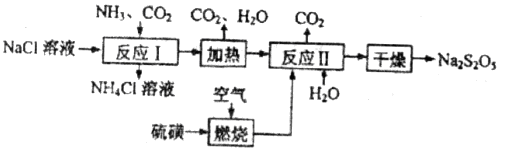
��֪����Ӧ����� �ȶಽ��Ӧ��
�ȶಽ��Ӧ��
��1����Ӧ��Ļ�ѧ����ʽΪ____________����Ӧ�����ʱӦ��ͨ��__________���塣
��2�����ȼ��ǰ�ȼ��ȳ�Һ̬��ͨ�������������¯�У�Ŀ����__________�����������������п�ѭ��ʹ�õ�������_____________��
��3����Ӧ��������Ʋμӷ�Ӧ���������������ʵ���֮�Ƚӽ�____________�������������㣬��ᵼ��_______________��
��4�� ��ϡ���ᷴӦ�ų�
��ϡ���ᷴӦ�ų� �������ӷ���ʽΪ___________��
�������ӷ���ʽΪ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ������п����Ҫ�ɷ�ΪZnS��������CdS��Fe2O3�����ʣ�Ϊԭ������ZnSO4��7H2O�Ĺ����������£�����֪Cd�Ľ�����Խ���Zn��Fe֮�䣩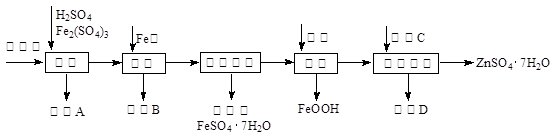
��1��������A�пɻ��һ�ֵ���ɫ�ǽ������ʵĸ���Ʒ���仯ѧʽΪ ��
��2����ȡ������Fe2(SO4)3�������� ����ȡʱFe2(SO4)3��ZnS������Ӧ�Ļ�ѧ����ʽΪ ��
��3���������̿�����Һ��pH��5.4���ң��÷�Ӧ�����ӷ���ʽΪ ���ù����ڿ�����ڴ������һ��������ԡ��ͷ��װ�ã���Ŀ���� ��
��4���û������ؽ���������Cd2+����������CΪ ��
��5������п���ܽ�����¶�֮��Ĺ�ϵ���±���
| �¶�/�� | 0 | 20 | 40 | 60 | 80 | 100 |
| �ܽ��/g | 41.8 | 54.1 | 70.4 | 74.8 | 67.2 | 60.5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�ҹ����������ձ��ȹ��Ҷ������Ƴ�һ���մɲ��ͻ������ֲ��ͻ��ķ���������������������һ�������Ҳ��״��ȵIJ���������ģ����ֲ�����(����)��
| A���������մ� | B���������մ� |
| C�����ά | D�������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com