
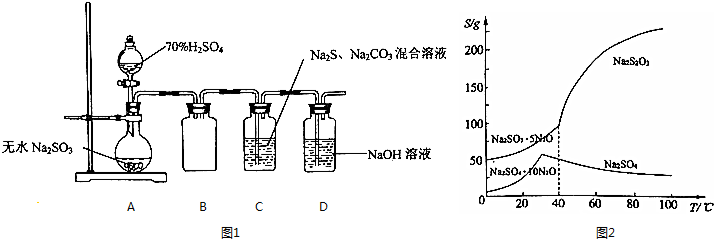
���� Aװ���Ʊ���������Cװ���кϳ�Na2S2O3���ᵼ��װ������ѹ��С��Bװ��Ϊ��ȫƿ���ã����������ж�������Ⱦ������������������Һ����ʣ��Ķ�������
��1�����ɵ������Ƹ������������Ʊ��棻Bװ�÷�ֹ������
��2���������Խ�����������������ˮ����������⣻
��3��C��SO2��Na2S��Na2CO3��Ӧ����Na2S2O3��ͬʱ���ɶ�����̼�����Ʒ�Ӧ������¶�Ϊ35�棬����ˮ�ķе㣬��ȡˮԡ���ȣ�
��4����ȡ�ϲ���Һ�����Ȼ�����Һ�����Ƿ����������
����ͼ��֪��30��֮ǰ������ܽ�����¶����߶�������30�����¶����߶����ͣ������������Ƶ��ܽ�����¶����߶�����ȡ�ؽᾧ�ķ��������ᴿ��ע�����Ҵ�ϴ�ӣ������ܽ�µ���ʧ��
��� �⣺Aװ���Ʊ���������Cװ���кϳ�Na2S2O3���ᵼ��װ������ѹ��С��Bװ��Ϊ��ȫƿ���ã����������ж�������Ⱦ������������������Һ����ʣ��Ķ�������
��1�����ɵ������Ƹ������������Ʊ��棬ʹ��Ӧ���ʱ�����Bװ����ȫƿ����ֹ������
�ʴ�Ϊ�����ɵ������ƹ��帽�����������Ʊ��棻��ֹ������
��2���������������Խ�����������ΪS�����ֻ�������������ˮ���������⣬�����������ݳ����ʴ�Ϊ���������������Խ�����������ΪS��������ˮ���������⣻
��3��C��SO2��Na2S��Na2CO3��Ӧ����Na2S2O3��ͬʱ���ɶ�����̼����Ӧ���ӷ���ʽΪ��4SO2+2S2-+CO32-=3S2O32-+CO2�����Ʒ�Ӧ������¶�Ϊ35�棬����ˮ�ķе㣬��ȡˮԡ���ȣ����Ⱦ��ȣ����ڿ����¶ȣ�
�ʴ�Ϊ��4SO2+2S2-+CO32-=3S2O32-+CO2��ˮԡ���ȣ�
��4���ټ���Na2S2O3•5H2O�ֲ�Ʒ���Ƿ���Na2SO4�ķ���Ϊ��ȡ�����ֲ�Ʒ��������ϡ���ᣬ���ã�ȡ�ϲ���Һ���μ��Ȼ�����Һ�������ְ�ɫ������˵������Na2SO4���ʣ�
�ʴ�Ϊ�����ã�ȡ�ϲ���Һ���μ��Ȼ�����Һ�������ְ�ɫ������˵������Na2SO4���ʣ�
����ͼ��֪��30��֮ǰ������ܽ�����¶����߶�������30�����¶����߶����ͣ������������Ƶ��ܽ�����¶����߶����ֲ�Ʒ������Na2SO4���ʣ����ᴿ��Ʒ��ʵ�鷽��Ϊ������Ʒ������������ˮ�У�����Ũ������ȴ��30��ᾧ�����ˣ�����ˮ�Ҵ�ϴ�ӣ����¸��
�ʴ�Ϊ������Ʒ������������ˮ�У�����Ũ������ȴ��30��ᾧ�����ˣ�����ˮ�Ҵ�ϴ�ӣ����¸��
���� ���⿼�������Ʊ�ʵ�顢���ʵķ����ᴿ��ʵ�鷽����Ƶȣ��ؼ��Ƕ�ԭ�������⣬ע������ȫ��ʶ�뻷����ʶ����������Ԫ�ػ�����֪ʶ��ʵ���Ʊ�����ԭ����Ŀ�Ѷ��еȣ�


 ����������������ϵ�д�
����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ʼ��� | B�� | ������ | C�� | ������ | D�� | ���ж� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | Y������������Ӧˮ��������Ա�X���� | |
| B�� | Zλ��Ԫ�����ڱ��еڶ����ڣ��ڢ�A�� | |
| C�� | X����̬�⻯����ȶ��Ա�Z���� | |
| D�� | M��ԭ�Ӱ뾶��Y��ԭ�Ӱ뾶�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������������ˮ�⣬ֻ���������� | |
| B�� | ʯ�͵��ѽ⡢ú�ĸ����ǻ�ѧ�仯 | |
| C�� | 1L1mol•L-1AlCl3��Һ�к�Al3+��ĿΪ6.02��1023 | |
| D�� | �����������۰�a��b����;����ȫת����;��a��;��b���ĸ����NaOH ;��a��Al$��_{��ȼ}^{O_{2}}$Al2O3$\stackrel{NaOH��Һ}{��}$NaAlO2��;��b��Al$\stackrel{NaOH��Һ}{��}$NaAlO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1mol�������ƺ�����ˮ��ַ�Ӧ��������ת�Ƶĵ�����ԼΪ2��6.02��1023 | |
| B�� | �繤�����н�������ͭ��ֱ���������ᵼ��ͭ�߸��챻���� | |
| C�� | MnS����Һ�еμ�����CuSO4��Һ������CuS��������Ksp��CuS����Ksp��MnS�� | |
| D�� | 0.1mol•L-1������ҺpH=a��0.01mol•L-1������ҺpH=b����a+1��b |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��� | ��Ӧ �¶�/�� | c��H2O2��/ mol•L-1 | V��H2O2�� /mL | m��MnO2�� /g | t/min |
| 1 | 20 | 2 | 10 | 0 | t1 |
| 2 | 20 | 2 | 10 | 0.1 | t2 |
| 3 | 20 | 4 | 10 | 0.1 | t3 |
| 4 | 40 | 2 | 10 | 0.1 | t4 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| �������� | Fe��OH��3 | Fe��OH��2 | Al��OH��3 | Ni��OH��2 |
| ��ʼ������pH | 1.1 | 6.5 | 3.5 | 7.1 |
| ������ȫ��pH | 3.2 | 9.7 | 4.7 | 9.2 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1mol��ŵ�����ӿ���7molH2��ȫ��Ӧ | |
| B�� | ����FeCl3 ��Һ��������ˮ����Ͷ����������� | |
| C�� | ��ŵ��������NaOH ��Һ���ȣ�������������ˮ�����ƺͶ������������� | |
| D�� | ����ˮ����Ͷ����������Ӿ�����NaHCO3 ��Һ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH3COONa | B�� | CH3COOH��CH3COONa | ||
| C�� | CH3COONa��NaOH | D�� | CH3COOH��NaOH |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com