
�����й�ʵ���������ȷ����
A����ʪ���pH��ֽ�ⶨ��Һ��pH���ⶨ���ƫС������Һһ��������
B���к͵ζ�ʵ���У���ƿ������ˮϴ����ʹ�ã��ζ���������ˮϴ��������� ���ô�װҺ��ϴ��ʹ��
C������ˮ�����Һ©�������������Ҵ������ã��ܽ�����ȡ���Ҵ���
D������FeCl2���ʵ�FeCl3��Һ��ͨ������C12��ּ������ɣ��ɵõ�������FeCl3����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Cl��Cl�����������У���ͬ���� �� ��
A����ѧ���ʡ���B����������� C������������ ��D��������Ӳ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ͭ�����ֳ�����������CuO��Cu2O��ijѧϰС��ȡ0.98 g(�þ�����ƽ����)Cu(OH)2���壬���������ͭ�����������ɣ����������¶ȱ仯��������ͼ1��ʾ��

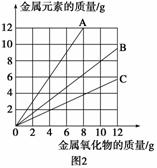
���⣬ijͬѧ������������ʾ����������������������Ԫ�������Ĺ�ϵ���ߣ���ͼ2��ʾ�������з�����ȷ���� (����)
A��ͼ1�в���a��b�Ļ�ѧʽ�ֱ�ΪCu2O��CuO
B��ͼ1���������й�����0.26 g H2O
C��ͼ2���������У���ʾCuO����������CuԪ�������Ĺ�ϵ����������A
D��ͼ2�л��ƴ�������߹�2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪Ksp(AgCl)��1.78��10��10��Ksp(Ag2CrO4)��2.00��10��12����ֻ����KCl��K2CrO4�Ļ����Һ�еμ�0.001 mol��L��1��AgNO3��Һ����AgCl��Ag2CrO4����ʱ�������Һ��CrO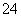 ��Ũ����5.000��10��3 mol��L��1����ʱ��Һ��Cl�������ʵ���Ũ����(����)
��Ũ����5.000��10��3 mol��L��1����ʱ��Һ��Cl�������ʵ���Ũ����(����)
A��1.36��10��5 mol��L��1���� B��8.90��10��6 mol��L��1
C��4.45��10��2 mol��L��1 D��1��10��5 mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D��E��Ϊ������ˮ�Ļ����������������¡�(����������������ɲ��ظ�)
| ������ | Na����Al3����Ag����Ba2����Fe3�� |
| ������ | Cl����CO |
�ֱ�ȡ��Һ��������ʵ�飺
����pH��ֽ�ֱ���B��C��Һ���ʼ��ԣ���0.1 mol/L B��ҺpH>13��
��D��Һ����ͭ�ۣ���Һ�������ӣ�
����E��Һ�мӹ���B��û�г���������
����A��Һ����ε��백ˮ�����������ȳɰ�ɫ������������ܽ⡣
��ش��������⣺
(1)B�����ƣ�________��E�����ƣ�________��
(2)Cˮ��Һ�ʼ��Ե�ԭ��(�����ӷ���ʽ��ʾ)��________��0.1 mol/L��C��Һ�к�0.1 mol/L��NaHCO3��Һ�У������ӵ�������C��Һ________NaHCO3��Һ��(�>������������<��)
(3)D��Һ��________(����ԡ��������ԡ������ԡ�)������D��Һ�������ɣ��õ��Ĺ���Ӧ�ǣ�________________________��(�ѧʽ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 Ϊ��Ч�������������ػ�����ȡ��ʩ���ƴ����������о�����Ч���ƿ����еĵ������̼��������������ﺬ���Ե���Ϊ��Ҫ��
Ϊ��Ч�������������ػ�����ȡ��ʩ���ƴ����������о�����Ч���ƿ����еĵ������̼��������������ﺬ���Ե���Ϊ��Ҫ��
(1)������ȼ������ʱ������N2��O2�ķ�Ӧ��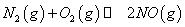 ���ǵ�������β���к���NO��ԭ��֮һ��
���ǵ�������β���к���NO��ԭ��֮һ��
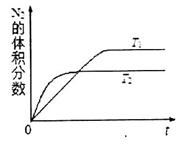
����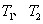 �¶��£�һ������NO�����ֽⷴӦʱN2�����������ʱ��仯����ͼ��ʾ������ͼ���жϷ�Ӧ
�¶��£�һ������NO�����ֽⷴӦʱN2�����������ʱ��仯����ͼ��ʾ������ͼ���жϷ�Ӧ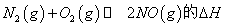 __________0(�>����<��)��
__________0(�>����<��)��
����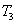 �¶��£���2L�ܱ������г���10molN2��5mo1O2��50���ﵽƽ�⣬���NO�����ʵ���Ϊ2mol����÷�Ӧ������
�¶��£���2L�ܱ������г���10molN2��5mo1O2��50���ﵽƽ�⣬���NO�����ʵ���Ϊ2mol����÷�Ӧ������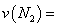 ___________________�����¶��£�����ʼʱ�����������г���N2��O2��Ϊ1 mol����ﵽƽ���N2��ת����Ϊ____________��
___________________�����¶��£�����ʼʱ�����������г���N2��O2��Ϊ1 mol����ﵽƽ���N2��ת����Ϊ____________��
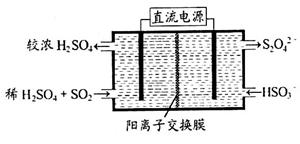 (2)������ͼ��ʾװ��(�缫��Ϊ���Ե缫)������SO2���������ų�����Һ������NO2��
(2)������ͼ��ʾװ��(�缫��Ϊ���Ե缫)������SO2���������ų�����Һ������NO2��
�������ĵ缫��ӦʽΪ_____________________��
���ڼ��������£��������ų�����Һ����NO2��ʹ��ת��Ϊ�����壬ͬʱ��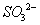 ���ɡ��÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ_______________��
���ɡ��÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ_______________��
(3)һ�������¿��ü״���CO��Ӧ���ɴ�������CO��Ⱦ�������£���a mol��L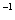 �Ĵ�����b mol
�Ĵ�����b mol L
L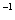
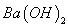 ��Һ�������ϣ���ַ�Ӧ����Һ�д���
��Һ�������ϣ���ַ�Ӧ����Һ�д���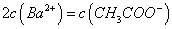 ����û����Һ�д���ĵ��볣��Ka=______________________(�ú�a��b�Ĵ���ʽ��ʾ)��
����û����Һ�д���ĵ��볣��Ka=______________________(�ú�a��b�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����Ѷ�������Һ�����ж��ԣ����뼡�����������һ���С����������
֮�ƣ������й�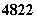 Ti��
Ti��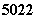 Ti ��˵������ȷ����
Ti ��˵������ȷ����
A.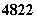 Ti��
Ti��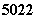 Ti����������ͬ������Ϊͬλ��
Ti����������ͬ������Ϊͬλ��
B.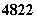 Ti��
Ti��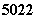 Ti����������ͬ������ͬλ��
Ti����������ͬ������ͬλ��
C.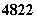 Ti��
Ti��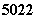 Ti����������ͬ����ͬһ�ֺ���
Ti����������ͬ����ͬһ�ֺ���
D.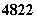 Ti��
Ti��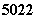 Ti�����������ͬ����������ͬ�����ܻ���Ϊͬλ��
Ti�����������ͬ����������ͬ�����ܻ���Ϊͬλ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ʵ���֮��Ϊ2��5��п��ϡ����ǡ����ȫ��Ӧ�������ᱻ��ԭ�IJ���ΪN2O��
��Ӧ������пû��ʣ�࣬��÷�Ӧ�б���ԭ��������δ����ԭ����������ʵ���֮����
A. 1��4 B.1��5 C. 2��3 D.2��5
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��״���£���3.36 L CO2����ͨ��200 mL 1.00 mol��L��1 NaOH��Һ�У���ַ�Ӧ����Һ��c(CO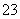 )�� c(HCO
)�� c(HCO )�ı�ֵΪ(������CO
)�ı�ֵΪ(������CO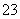 ��HCO
��HCO ��ˮ��) (����)
��ˮ��) (����)
A��1��1 B��1��2
C��2��1 D��1��3
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com