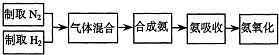
A��������
SO2
SO2
��
����������
����������
��ɢ�������У��ڿ���������������·���һϵ�б仯������ʱ����ˮ���գ�����ˮ��pHС��
5.6
5.6
ʱ���γ������꣮
ijУ�Ƽ�С���ͬѧ�Dzɼ�������Ʒ��ÿ��һ��ʱ��ⶨ��Ʒ��pH���õ��������ݣ�
| ʱ�� |
��ʼ |
8h�� |
16h�� |
24h�� |
32h�� |
40h�� |
48h�� |
| pH |
5.0 |
4.8 |
4.5 |
4.3 |
4.2 |
4.0 |
4.0 |
�ش��������⣺
��1���������ʱ��pH������õ���Ҫԭ��Ϊ
��ˮ�������ᱻ���������ᣬ������ǿ
��ˮ�������ᱻ���������ᣬ������ǿ
��
��2��ú�к�������̬������д��������һϵ�б仯�����������Ҫ�ɷֵ��йػ�ѧ����ʽ�������߲�һ��������
��
2SO2+O2=2SO3
2SO2+O2=2SO3
��
SO3+H2O�TH2SO4
SO3+H2O�TH2SO4
��
��
��
��
B����A��B�����������ǵ������ͬ����Լ��85.7%��̼����A������������ܶ���28����Bʽ���ȿ�����ƽ��ʽ����С�������ʽ��A��ͬ����A��B����ʹ������Ȼ�̼��Һ��ɫ����������ʵ����ʵ���㲢�ش����⣮
��1������A��B�����Ļ�ѧʽ�������㲽��д�����棩
A
C4H8
C4H8
��B
C2H4
C2H4
��
��2��A��B��
C4H8
C4H8
����A��B�ķ���ʽ������ͬ���칹�壮
��3��д��B��������Ȼ�̼��Һ��Ӧ�Ļ�ѧ����ʽ��
CH2=CH2+Br2��CH2Br-CH2Br
CH2=CH2+Br2��CH2Br-CH2Br
��