
�������ӷ���ʽ�У�������ȷ���� �� ��
A���� NaHS ��Һ��ͨ������������HS+Cl2 =S��+H��+2Cl
B����Fe��OH��3����Һ�м�������Fe��OH��3+3H��=Fe3��+3H2O
C���� 10 mL 0.1 mol•L��l HCl��Һ��20 mL 0.05 mol•L��l Na3PO4 ��Һ���
3H��+2 PO43= H2PO4+HPO42
D���� Mg��HCO3��2��Һ�м������NaOH ��Һ
Mg2��+2HCO3+2OH=MgCO3��+CO32+2H2O

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | SO42- | Mg2+ | Fe3+ | Na+ | Cl- |
| Ũ�ȣ�mol/L�� | a | 0.05 | 0.10 | 0.50 | 0.58 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��ѧʽ | ���볣�� |
| HClO | K=3��10-8 |
| H2CO3 | K1=3��10-3 |
| K2=3��10-11 |
| A����Na2CO3��Һ�еμ�������ˮ��CO32-+2Cl2+H2O=2Cl-+2HClO+CO2 |
| B����NaHCO3��Һ�еμ�������ˮ��2HCO3-+CI2=CI-+CIO-+2CO2+H2O |
| C����NaClO��Һ��ͨ������CO2��CO2+NaClO+H2O=NaHCO3+HClO |
| D����NaClO��Һͨ������CO2��CO2+2NaClO+H2O=Na2CO3+2HClO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
����� ÿƬ������������ ����Ӧ֢�� ����ȱ����ƶѪ֢��Ԥ���������� �������÷��� ����Ԥ���� �����ء� �ܹ⡢�ܷ⡢�ڸ��ﴦ���� |
(1)��ҩƷ��Fe2+�Ỻ�����������ҹ涨��ҩ����Fe2+�������ʳ���10.00%�������ٷ��á�
��Ϊ�˼���ijҩ����۵ġ������ơ��Ƿ�������ʵ����Ӧѡ�õļ����Լ�Ϊ__________(���Լ�������)��
��ʵ���ҿɲ���H2SO4�ữ��KMnO4��Һ���ԡ������ơ��е�Fe2+���еζ�(����ҩƷ�У������ɷֲ���KMnO4��Ӧ)������ƽ�������ӷ���ʽ��
__________![]() +__________Fe2++__________H+
+__________Fe2++__________H+![]() __________Mn2++__________Fe3++__________H2O
__________Mn2++__________Fe3++__________H2O
�۳�����������Ԫ����������Ϊ20.00%�ġ������ơ�
(2)��֪������������Է�������Ϊ172��������Ϊ�л��ᡣ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ���Ϻ��г����ظ�����ѧ����ĩ��һģ�����Ի�ѧ�Ծ��������棩 ���ͣ������
��������ĵ���ƽ�ⳣ�����±���
|
���� |
HCOOH |
HClO |
H2CO3 |
H2SO3 |
|
����ƽ�ⳣ�� ��25�棩 |
|
|
|
|
��1�����¶���ͬʱ���������Kiֵ�����Ե����ǿ���Ĺ�ϵΪ��________________________��
��2���������ӷ���ʽ��ȷ����
A��2ClO�� + H2O + CO2 �� 2HClO + CO32��
B��2HCOOH + CO32�� �� 2HCOO�� + H2O + CO2��
C��H2SO3 + 2HCOO�� �� 2HCOOH + SO32��
D��Cl2 + H2O+2CO32�� �� 2HCO3�� + Cl�� + ClO��
��3�������£�pH=3��HCOOH��Һ��pH=11��NaOH��Һ�������Ϻ���Һ������Ũ���ɴ�С��˳��Ϊ������������������������������������������������������������������
������(H2SeO3)Ҳ��һ�ֶ�Ԫ���ᣬ��������һ����ɫ���壬������ˮ���н�ǿ�������ԡ�
��4������������Һ�в���ͨ��SO2 ��������ɫ���ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ������������������������ ��������������������������������������������
��5������������30%��H2O2���ȿ��Ƶ�����(H2SeO4)����Ӧ����ʽ���£�
H2SeO3 + H2O2 �� H2SeO4+H2O������˵������ȷ���ǡ���������������������������������
A��H2O2�������������ǻ�ԭ��
B��H2O �Ȳ������������ֲ��ǻ�ԭ����
C��H2SeO4���������������ǻ�ԭ����
D�������ԣ�H2SeO3��H2SeO4
���ᣨH6TeO6����һ�ֺ������ᣬ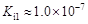 �������������Ա����ỹҪǿ�������Խ����У�����ɽ�HI������I2������ʽ���£�
�������������Ա����ỹҪǿ�������Խ����У�����ɽ�HI������I2������ʽ���£�
���� ��HI+���� ��H6TeO6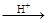 ���� ��TeO2+�� ����Te+���� ��I2+�� ����H2O
���� ��TeO2+�� ����Te+���� ��I2+�� ����H2O
��6������Ӧ�����ɵ�TeO2��Te�����ʵ���֮��Ϊ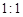 ������ƽ������ѧ����ʽ��
������ƽ������ѧ����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ʡ�����и�����һ���ʼ컯ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ӷ���ʽ��ȷ����
A��������������Һ��ȥ��Ƭ�����ϵ�����Ĥ��Al3����4OH��=AlO2����2H2O
B���ں�3��2amolHNO3��ϡ��Һ�У�����amol���ۣ�5Fe��4NO3����16H��=3Fe2����2Fe3����4NO����8H2O
C������ʯ��ˮ�������С�մ���Һ��Ӧ��Ca2����OH����HCO3��=CaCO3����H2O
D������������ʹ����KMnO4��Һ��ɫ��3H2O2+2MnO4-+6H+=2Mn2++4O2��+6H2O
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com