
| 10-4��10-4 |
| 0.1 |
| 10-14 |
| 10-4 |
| 10-4 |
| 10-10 |
| 1��10-13 |
| 1��10-11 |
| bL��0.1mol/L-aL��0.01mol/L |
| aL+bL |


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Na+ NH4+ S2- AlO2- |
| B��Fe3+ K+ HSO3- Cl- |
| C��NH4+ Ca2+ HCO3-AlO2- |
| D��Fe2+ Mg2+ SO42- NO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������£�30 g��2-�����к����ǻ�����ĿΪ0.5 NA |
| B��15g C2H6��C-H����ĿΪ3NA |
| C��28g��ϩ���ϩ�Ļ�����壬��̼ԭ������Ϊ2NA |
D��7.8g �к��е�̼̼˫����ĿΪ0.3NA �к��е�̼̼˫����ĿΪ0.3NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
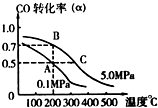 ��10L�ܱ������г���10mol CO ��20mol H2���ڴ��������·�Ӧ���ɼ״���
��10L�ܱ������г���10mol CO ��20mol H2���ڴ��������·�Ӧ���ɼ״����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
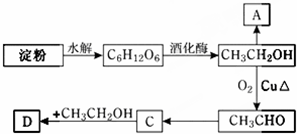
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
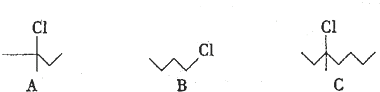
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
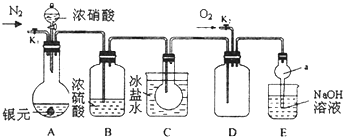
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com