
ЗДгІSO2(g)+ NO2(g)SO3(g)+NO(g) ,ШєдквЛЖЈЮТЖШЯТЃЌНЋЮяжЪЕФСПХЈЖШОљЮЊ2mol/LЕФSO2(g)КЭNO2(g)зЂШывЛУмБеШнЦїжаЃЌЕБДяЕНЦНКтзДЬЌЪБЃЌВтЕУШнЦїжаSO2(g)ЕФзЊЛЏТЪЮЊ50%ЃЌЪдЧѓЃКдкИУЮТЖШЯТЁЃ
ЃЈ1ЃЉДЫЗДгІЕФЦНКтГЃЪ§ЁЃ
ЃЈ2ЃЉШєSO2(g)ЕФГѕЪМХЈЖШдіДѓЕН3mol/L, NO2(g)ЕФГѕЪМХЈЖШШдЮЊ2mol/LЃЌдђSO2(g)ЁЂNO2(g)ЕФзЊЛЏТЪБфЮЊЖрЩйЃП

| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЛЏбЇЁЊЁЊЛЏбЇгыММЪѕЁПЃЈ15ЗжЃЉСђЫсЙЄвЕЩњВњгІПМТЧзлКЯОМУаЇвцЮЪЬтЁЃ
ЃЈ1ЃЉШєДгЯТСаЫФИіГЧЪажабЁдёвЛДІаТНЈвЛзљСђЫсГЇЃЌФуШЯЮЊГЇжЗвЫбЁдк
ЕФНМЧјЃЈЬюбЁЯюЕФБъКХЃЉ
AЃЎгаЗсИЛЛЦЬњПѓзЪдДЕФГЧЪа BЃЎЗчЙтауРіЕФТУгЮГЧЪа
CЃЎЯћКФСђЫсНЯЖрЕФЙЄвЕГЧЪа DЃЎШЫПкГэУмЕФЮФЛЏЁЂЩЬвЕжааФГЧЪа
ЃЈ2ЃЉОнВтЫуЃЌНгДЅЗЈжЦСђЫсЙ§ГЬжаЃЌШєЗДгІШШЖМЮДБЛРћгУЃЌдђУПЩњВњ1t 98%СђЫсашЯћКФ3.6ЁС105kJФмСПЁЃШєЗДгІ:SO2(g)+1/2O2(g)=SO3(g) ЁїH=Ѓ98ЃЎ3kJЁЄmolЃ1ЃЛЗХГіЕФШШСПФмдкЩњВњЙ§ГЬжаЕУЕНГфЗжРћгУ, ЧыЭЈЙ§МЦЫуХаЖЯУПЩњВњ1t98%СђЫсжЛашЭтНчЬсЙЉЃЈЛђПЩЯђЭтНчЪфГіЃЉ ЧЇНЙФмСПЃЛ
ЃЈ3ЃЉCuFeS2ЪЧЛЦЬњПѓЕФСэвЛГЩЗжЃЌьбЩеЪБЃЌCuFeS2зЊЛЏЮЊCuOЁЂFe2O3КЭSO2ЃЌИУЗДгІЕФЛЏбЇЗНГЬЪНЮЊ ЁЃ
ЃЈ4ЃЉгЩСђЫсГЇЗаЬкТЏХХГіЕФПѓдќжаКЌгаFe2O3ЁЂCuOЁЂCuSO4ЃЈгЩCuOгыSO3дкЗаЬкТЏжаЛЏКЯЖјГЩЃЉЃЌЦфжаСђЫсЭЕФжЪСПЗжЪ§ЫцЗаЬкТЏЮТЖШВЛЭЌЖјБфЛЏЃЈМћЯТБэЃЉ
| ЗаЬкТЏЮТЖШ/Ёц | 600 | 620 | 640 | 660 |
| ПѓдќжаCuSO4ЕФжЪСПЗжЪ§/% | 9.3 | 9.2 | 9.0 | 8.4 |
вбжЊCuSO4дкЕЭгк660ЁцЪБВЛЛсЗжНтЃЌЧыМђвЊЗжЮіЩЯБэжаCuSO4ЕФжЪСПЗжЪ§ЫцЮТЖШЩ§ИпЖјНЕЕЭЕФдвђ ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2011ЁЊ2012бЇФъСЩФўЪЁЩђбєЖўжаИпЖўЩЯбЇЦк10дТдТПМЛЏбЇЪдОэ ЬтаЭЃКЬюПеЬт
ЃЈ12ЗжЃЉЯђОјШШКуШнУмБеШнЦїжаЭЈШыSO2КЭNO2ЃЌдквЛЖЈЬѕМўЯТЪЙЗДгІ
SO2(g)ЃЋNO2(g) SO3(g)ЃЋNO(g)ДяЕНЦНКтЃЌе§ЗДгІЫйТЪЫцЪБМфБфЛЏЕФЪОвтЭМШчЯТЫљЪОЁЃ
SO3(g)ЃЋNO(g)ДяЕНЦНКтЃЌе§ЗДгІЫйТЪЫцЪБМфБфЛЏЕФЪОвтЭМШчЯТЫљЪОЁЃ
ЃЈ1ЃЉЕуa-cЖЮЃЌЫйТЪБфЛЏЕФжївЊдвђЪЧ ЃЌЕуcКѓЫйТЪБфЛЏЕФжївЊдвђЪЧ ЁЃ
ЃЈ2ЃЉcЕуЪЧЗёвбОДяЕНЦНКтзДЬЌ ЃЈЬюЁАЪЧЁБЁЂЁАЗёЁБЛђЁАВЛвЛЖЈЁБЃЉ
ЃЈ3ЃЉЗДгІЮяХЈЖШЃКaЕу bЕуЃЈЬюЁА<ЁБЁЂЁА=ЁБЛђЁА>ЁБЃЉ
ЃЈ4ЃЉЗДгІЮяЕФзмФмСП ЩњГЩЮяЕФзмФмСПЃЈЬюЁА<ЁБЁЂЁА=ЁБЛђЁА>ЁБЃЉ
ЃЈ5ЃЉЁїt1ЃНЁїt2ЪБЃЌSO2ЮяжЪЕФСПЕФБфЛЏСПЃКaЁЋbЖЮ bЁЋcЖЮЃЈЬюЁА<ЁБЁЂЁА=ЁБЛђЁА>ЁБЃЉ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2013-2014бЇФъНЮїЪЁИгжнЪаЪЎЖўЯиИпЖўЩЯбЇЦкЦкжаПМЪдЛЏбЇЪдОэЃЈНтЮіАцЃЉ ЬтаЭЃКЬюПеЬт
ЃЈ1ЃЉвбжЊКьСзБШАзСзЮШЖЈЃЌгжжЊЃК4P(АзСзЃЌsЃЉ+5O2ЃЈgЃЉЃН2P2O5ЃЈsЃЉ ЁїH1ЃЛ
4PЃЈКьСзЃЌsЃЉ+5O2ЃЈgЃЉЃН2P2O5ЃЈsЃЉ ЁїH2ЃЌдђІЄH1КЭІЄH2ЕФЙиЯЕЪЧЁїH1 ЁїH2ЃЈЬюЁАЃОЁБЁЂЁАЃМЁБ
ЛђЁАЃНЁБЃЉЁЃ
ЃЈ2ЃЉвбжЊH2ЃЈgЃЉКЭCH3OHЃЈlЃЉЕФШМЩеШШЁїHЗжБ№ЮЊ-285.8kJЁЄmol-1КЭ-726.5kJЁЄmol-1ЃЌаДГігЩCO2
КЭH2ЩњГЩвКЬЌМзДМКЭвКЬЌЫЎЕФШШЛЏбЇЗНГЬЪН ЁЃ
ЃЈ3ЃЉвбжЊвЛЖЈЮТЖШЯТЃЌЯТСаЗДгІЕФЦНКтГЃЪ§ЃКSO2(g)+1/2O2(g) 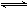 SO3(g)
K1,CO(g)+1/2O2(g)
SO3(g)
K1,CO(g)+1/2O2(g)
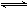 CO2(g)
K2ЁЃдђЯрЭЌЮТЖШЯТЗДгІSO2(g)+CO2(g)
CO2(g)
K2ЁЃдђЯрЭЌЮТЖШЯТЗДгІSO2(g)+CO2(g) 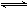 SO3(g)+CO(g)ЕФЦНКтГЃЪ§ЮЊ
ЁЃ
SO3(g)+CO(g)ЕФЦНКтГЃЪ§ЮЊ
ЁЃ
ЃЈгУK1ЁЂK2БэЪОЃЉ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2012ФъТГПЦАцИпжаЛЏбЇбЁао6 2ЗДгІЬѕМўЖдЛЏбЇЗДгІЕФгАЯьСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ЗДгІSO2(g)ЃЋNO2(g)  NO(g)ЃЋSO3(g)дквЛЖЈЬѕМўЯТНЈСЂЦНКтЃЌдйМгШывЛЖЈСПЕФO2ЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧ(ЁЁЁЁ)
NO(g)ЃЋSO3(g)дквЛЖЈЬѕМўЯТНЈСЂЦНКтЃЌдйМгШывЛЖЈСПЕФO2ЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧ(ЁЁЁЁ)
AЃЎЦНКтзѓвЦЃЌШнЦїФкбЙЧПвЛЖЈдіДѓ
BЃЎЦНКтгввЦЃЌДяЕНЦНКтЪБШнЦїбЙЧПвЛЖЈдіДѓ
CЃЎЦНКтВЛвЛЖЈЗЂЩњвЦЖЏЃЌШнЦїФкбЙЧПвЛЖЈдіДѓ
DЃЎЦНКтгввЦЃЌSO2ЕФзЊЛЏТЪЬсИп
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2010ФъКўББЪЁИпЖўЩЯбЇЦкЦкжаПМЪдЪдЬтЛЏбЇ ЬтаЭЃКбЁдёЬт
ЗДгІSO2(g)
+ NO2(g)  NO(g) + SO3(g)дквЛЖЈЬѕМўЯТНЈСЂЦНКтЃЌдйМгШывЛЖЈСПЕФO2ЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧ ЃЈ
ЃЉ
NO(g) + SO3(g)дквЛЖЈЬѕМўЯТНЈСЂЦНКтЃЌдйМгШывЛЖЈСПЕФO2ЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧ ЃЈ
ЃЉ
AЃЎЦНКтзѓвЦЃЌШнЦїФкбЙЧПВЛвЛЖЈдіДѓ
BЃЎЦНКтгввЦЃЌДяЕНЦНКтЪБШнЦїФкбЙЧПвЛЖЈдіДѓ
CЃЎ ЦНКтгввЦЃЌSO2ЕФзЊЛЏТЪЬсИп
DЃЎЦНКтВЛвЛЖЈвЦЖЏЃЌШнЦїФкбЙЧПвЛЖЈдіДѓ
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com