
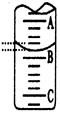
ijѧÉúÓĂŇŃÖŞÎďÖĘľÄÁżĹ¨śČľÄŃÎËáŔ´˛âś¨Î´ÖŞÎďÖĘľÄÁżĹ¨śČľÄNaOHČÜҺʹŁŹŃĄÔńź×ťůłČ×÷ָʞźÁĄŁÇëĚîĐ´ĎÂÁпհףş
Ł¨1ŁŠÓĂąęןľÄŃÎËáľÎś¨´ý˛âľÄNaOHČÜҺʹŁŹ×óĘÖÎŐËáĘ˝ľÎś¨šÜľÄťîČűŁŹÓŇĘÖŇĄśŻ×śĐÎĆżŁŹŃŰžŚ×˘ĘÓ ŁŹÖąľ˝×îşóźÓČëŇťľÎŃÎËáşóŁŹČÜŇşÓÉ ÉŤąäÎŞ ŁŹÇŇ ÎŞÖšĄŁ
Ł¨2ŁŠĎÂÁвŮ×÷ÖĐżÉÄÜĘšËů˛âNaOHČÜŇşľÄŨśČĘý־ƍľÍľÄĘÇ________
| AŁŽËáĘ˝ľÎś¨šÜδÓĂąęןŃÎËáČóĎ´žÍÖą˝ÓעČëąęןŃÎËá |
| BŁŽľÎś¨Ç°Ę˘ˇĹNaOHČÜŇşľÄלĐÎĆżÓĂŐôÁóËŽĎ´žťşóĂťÓиÉÔď |
| CŁŽËáĘ˝ľÎś¨šÜÔھΜ¨Ç°ÓĐĆřĹÝŁŹľÎś¨şóĆřĹÝĎűʧ |
| DŁŽśÁČĄŃÎËáĚĺťýĘąŁŹżŞĘźŃöĘÓśÁĘýŁŹľÎś¨˝áĘřĘą¸ŠĘÓśÁĘý |

| ľÎś¨ ´ÎĘý | ´ý˛âNaOHČÜŇşľÄĚĺťý/mL | 0ŁŽ100 mol/LŃÎËáľÄĚĺťý/mL | ||
| ľÎś¨Ç°żĚśČ | ľÎś¨şóżĚśČ | ČÜŇşĚĺťý/mL | ||
| ľÚŇť´Î | 25ŁŽ00 | 0ŁŽ20 | 20ŁŽ22 | |
| ľÚśţ´Î | 25ŁŽ00 | 0ŁŽ56 | 24ŁŽ54 | |
| ľÚČý´Î | 25ŁŽ00 | 0ŁŽ42 | 20ŁŽ40 | |
לĐÎĆżÄÚČÜŇşŃŐÉŤľÄąäťŻŁ¨1ˇÖŁŠ ťĆÉŤŁ¨1ˇÖŁŠ łČÉŤŁ¨1ˇÖŁŠ°ëˇÖÖÓ˛ťąäÉŤŁ¨1ˇÖŁŠ
Ł¨2ŁŠDŁ¨1ˇÖŁŠ Ł¨3ŁŠ20ŁŽ00mLŁ¨1ˇÖŁŠ Ł¨4ŁŠ0ŁŽ0800 mol/LŁ¨2ˇÖŁŠ
˝âÎöĘÔĚâˇÖÎöŁşŁ¨1ŁŠËáźîÖк;Μ¨ĘąŁŹŃŰžŚŇŞ×˘ĘÓלĐÎĆżÄÚČÜŇşľÄŃŐÉŤąäťŻŁŹľÎś¨ÖŐľăĘąČÜŇşŃŐÉŤÓÉťĆÉŤÍťąäÎŞłČÉŤŁŹÇŇ°ëˇÖÖÓÄÚ˛ťÍĘÉŤŁŽŁ¨2ŁŠAĄ˘ËáĘ˝ľÎś¨šÜδÓĂąęןŃÎËáČÜŇşČóĎ´žÍÖą˝ÓעČëąęןŃÎËáČÜŇşŁŹąęןҺľÄŨśČĆŤĐĄŁŹÔěłÉVŁ¨ąęןŁŠĆŤ´óŁŹ¸ůžÝcŁ¨´ý˛âŁŠ=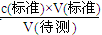 żÉÖŞŁŹ˛âś¨cŁ¨NaOHŁŠĆŤ´óŁŹšĘA˛ťˇűşĎĄŁBĄ˘ľÎś¨Ç°Ę˘ˇĹÇâŃőťŻÄĆČÜŇşľÄלĐÎĆżÓĂŐôÁóËŽĎ´žťşóĂťÓиÉÔ´ý˛âŇşľÄÎďÖĘľÄÁż˛ťąäŁŹśÔVŁ¨ąęןŁŠÎŢÓ°Ď죏¸ůžÝcŁ¨´ý˛âŁŠ=
żÉÖŞŁŹ˛âś¨cŁ¨NaOHŁŠĆŤ´óŁŹšĘA˛ťˇűşĎĄŁBĄ˘ľÎś¨Ç°Ę˘ˇĹÇâŃőťŻÄĆČÜŇşľÄלĐÎĆżÓĂŐôÁóËŽĎ´žťşóĂťÓиÉÔ´ý˛âŇşľÄÎďÖĘľÄÁż˛ťąäŁŹśÔVŁ¨ąęןŁŠÎŢÓ°Ď죏¸ůžÝcŁ¨´ý˛âŁŠ=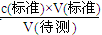 żÉÖŞŁŹ˛âś¨cŁ¨NaOHŁŠÎŢÓ°Ď죏šĘB˛ťˇűşĎŁťCŃĄĎîËáĘ˝ľÎś¨šÜÔھΜ¨Ç°ÓĐĆřĹÝŁŹľÎś¨şóĆřĹÝĎűʧŁŹÔěłÉVŁ¨ąęןŁŠĆŤ´óŁŹ¸ůžÝcŁ¨´ý˛âŁŠ=
żÉÖŞŁŹ˛âś¨cŁ¨NaOHŁŠÎŢÓ°Ď죏šĘB˛ťˇűşĎŁťCŃĄĎîËáĘ˝ľÎś¨šÜÔھΜ¨Ç°ÓĐĆřĹÝŁŹľÎś¨şóĆřĹÝĎűʧŁŹÔěłÉVŁ¨ąęןŁŠĆŤ´óŁŹ¸ůžÝcŁ¨´ý˛âŁŠ=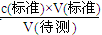 żÉÖŞŁŹ˛âś¨cŁ¨NaOHŁŠĆŤ´óŁŹšĘC˛ťˇűşĎŁťDŃĄĎśÁČĄŃÎËáĚĺťýĘąŁŹżŞĘźŃöĘÓśÁĘýŁŹľÎś¨˝áĘřĘą¸ŠĘÓśÁĘýŁŹÔěłÉVŁ¨ąęןŁŠĆŤĐĄŁŹ¸ůžÝcŁ¨´ý˛âŁŠ=
żÉÖŞŁŹ˛âś¨cŁ¨NaOHŁŠĆŤ´óŁŹšĘC˛ťˇűşĎŁťDŃĄĎśÁČĄŃÎËáĚĺťýĘąŁŹżŞĘźŃöĘÓśÁĘýŁŹľÎś¨˝áĘřĘą¸ŠĘÓśÁĘýŁŹÔěłÉVŁ¨ąęןŁŠĆŤĐĄŁŹ¸ůžÝcŁ¨´ý˛âŁŠ=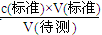 żÉÖŞŁŹ˛âś¨cŁ¨NaOHŁŠĆŤľÍŁŹšĘDˇűşĎŁťŁ¨3ŁŠĆđĘźśÁĘýÎŞ0ŁŽ10mLŁŹÖŐľăśÁĘýÎŞ20ŁŽ10mLŁŹŃÎËáČÜŇşľÄĚĺťýÎŞ20ŁŽ00mLĄŁŁ¨4ŁŠ¸ůžÝĘýžÝľÄÓĐЧĐÔŁŹÉáČĽľÚ2×éĘýžÝŁŹČťşóÇółö1Ą˘3×éĆ˝žůĎűşÄVŁ¨ŃÎËᣊ=20ŁŽ00mLŁŹ
żÉÖŞŁŹ˛âś¨cŁ¨NaOHŁŠĆŤľÍŁŹšĘDˇűşĎŁťŁ¨3ŁŠĆđĘźśÁĘýÎŞ0ŁŽ10mLŁŹÖŐľăśÁĘýÎŞ20ŁŽ10mLŁŹŃÎËáČÜŇşľÄĚĺťýÎŞ20ŁŽ00mLĄŁŁ¨4ŁŠ¸ůžÝĘýžÝľÄÓĐЧĐÔŁŹÉáČĽľÚ2×éĘýžÝŁŹČťşóÇółö1Ą˘3×éĆ˝žůĎűşÄVŁ¨ŃÎËᣊ=20ŁŽ00mLŁŹ
HCl +NaOH = NaCl +H2O
0ŁŽ0200LĄÁ0ŁŽ1000mol/L 0ŁŽ025LĄÁCŁ¨NaOHŁŠ
ÔňCŁ¨NaOHŁŠ=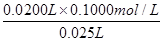 =0ŁŽ0800 mol/L
=0ŁŽ0800 mol/L
żźľăŁşËáźîÖк;Μ¨ŁŹÎďÖĘľÄÁżĹ¨śČźĆË㥣



| Äęźś | ¸ßÖĐżÎłĚ | Äęźś | łőÖĐżÎłĚ |
| ¸ßŇť | ¸ßŇťĂâˇŃżÎłĚÍĆźöŁĄ | łőŇť | łőŇťĂâˇŃżÎłĚÍĆźöŁĄ |
| ¸ßśţ | ¸ßśţĂâˇŃżÎłĚÍĆźöŁĄ | łőśţ | łőśţĂâˇŃżÎłĚÍĆźöŁĄ |
| ¸ßČý | ¸ßČýĂâˇŃżÎłĚÍĆźöŁĄ | łőČý | łőČýĂâˇŃżÎłĚÍĆźöŁĄ |
żĆÄżŁş¸ßÖĐťŻŃ§ Ŕ´Ô´Łş ĚâĐÍŁşĚîżŐĚâ
š¤ŇľÉĎŇÔČíĂĚżóÎŞÔÁĎŁŹŔűÓĂÉŐ˝áŃĚĆřÖĐľÄSO2ÖĆą¸MnSO4Ą¤H2OľÄÁ÷łĚČçĎÂŁş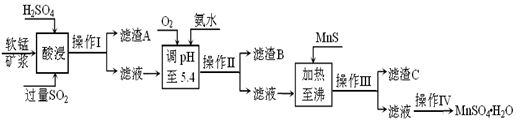
ÄłČíĂĚżóľÄÖ÷ŇŞłÉˇÖÎŞMnO2ŁŹťšşŹÓĐSi(16.27%)Ą˘Fe(5.86%)Ą˘Al(3.42%)Ą˘Zn(2.68%)şÍCuŁ¨0.86%ŁŠľČÔŞËؾĝŻşĎÎ北Ëá˝ţšýłĚˇ˘Éúˇ´ÓŚŁşMemOn+H+ĄúMeŁ¨2n/mŁŠ++H2OŁŹMeąíĘžFeĄ˘ AlĄ˘ ZnĄ˘ CuľČĄŁ25ĄćĘą˛żˇÖŃôŔë×ÓŇÔÇâŃőťŻÎďťňÁňťŻÎďľÄĐÎĘ˝ÍęČŤłÁľíĘąČÜŇşľÄpHźűĎÂąíŁş
| łÁľíÎď | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Mn(OH)2 | Cu(OH)2 | Zn(OH)2 | CuS | ZnS | MnS | FeS |
| pH | 5.2 | 3.2 | 9.7 | 10.4 | 6.7 | 8.0 | ĄÝ-0.42 | ĄÝ2.5 | ĄÝ7 | ĄÝ7 |
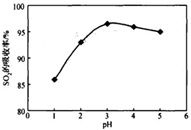
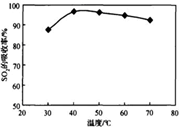
˛éż´´đ°¸şÍ˝âÎö>>
żĆÄżŁş¸ßÖĐťŻŃ§ Ŕ´Ô´Łş ĚâĐÍŁşĘľŃéĚâ
ÄłťŻŃ§ĘľŃéĐĄ×é´ÓĘĐłĄÉĎÂňŔ´ŇťĆżÄłĆˇĹĆĘłÓð״ף¨Ö÷ŇŞĘÇ´×ËáľÄËŽČÜŇşŁŠŁŹÓĂĘľŃéĘŇąęןNaOHČÜŇşśÔĆä˝řĐоΜ¨ŇԲ✨ËüľÄןȡŨśČĄŁĎÂąíĘÇ4ÖÖłŁźűָʞźÁľÄąäÉŤˇśÎ§Łş
| ָʞźÁ | ĘŻČď | ź×ťůłČ | ź×ťůşě | ˇÓĚŞ |
| ąäÉŤˇśÎ§Ł¨pHŁŠ | 5ŁŽ0ĄŤ8ŁŽ0 | 3ŁŽ1ĄŤ4ŁŽ4 | 4ŁŽ4ĄŤ6ŁŽ2 | 8ŁŽ2ĄŤ10ŁŽ0 |
| ĘľŃé´ÎĘý | ľÚŇť´Î | ľÚśţ´Î | ľÚČý´Î |
| ĎűşÄNaOHČÜŇşĚĺťý/mL | 26ŁŽ02 | 25ŁŽ35 | 25ŁŽ30 |
˛éż´´đ°¸şÍ˝âÎö>>
żĆÄżŁş¸ßÖĐťŻŃ§ Ŕ´Ô´Łş ĚâĐÍŁşĘľŃéĚâ
ÄłÉŐźîČÜŇşÖĐşŹÓĐÉŮÁżÔÓÖĘ(˛ťÓëŃÎËᡴӌ)ŁŹĎÖÓĂÖк;Μ¨˛âś¨ĆäŨśČĄŁ
Ł¨1ŁŠľÎś¨Łş˘ŮÓĂ Ę˝ľÎś¨šÜʢװc mol/LŃÎËáąęןҺĄŁČçÍźąíʞij´ÎľÎś¨Ęą50 mLľÎś¨šÜÖĐÇ°şóŇşĂćľÄÎťÖĂĄŁÇ뽍ÓĂČĽľÄąęןŃÎËáľÄĚĺťýĚîČë˘ŰąíżŐ¸ńÖĐŁŹ´ËĘąľÎś¨šÜÖĐŇşĚĺľÄĚĺťý mLĄŁ
˘ÚĎÂąíĘÇ4ÖÖłŁźűָʞźÁľÄąäÉŤˇśÎ§Łş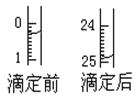
| ָʞźÁ | ĘŻČď | ź×ťůłČ | ź×ťůşě | ˇÓĚŞ |
| ąäÉŤˇśÎ§Ł¨pHŁŠ | 5ŁŽ0ĄŞ8ŁŽ0 | 3ŁŽ1ĄŞ4ŁŽ4 | 4ŁŽ4ĄŞ6ŁŽ2 | 8ŁŽ2ĄŞ10ŁŽ0 |
| ľÎś¨ĐňşĹ | ´ý˛âŇşĚĺťý(mL) | ËůĎűşÄŃÎËáąęןҺľÄĚĺťý(mL) | ||
| ľÎś¨Ç° | ľÎś¨şó | ĎűşÄľÄĚĺťý | ||
| 1 | V | 0ŁŽ50 | 25ŁŽ80 | 25ŁŽ30 |
| 2 | V | | | |
| 3 | V | 6ŁŽ00 | 31ŁŽ35 | 25ŁŽ35 |
˛éż´´đ°¸şÍ˝âÎö>>
żĆÄżŁş¸ßÖĐťŻŃ§ Ŕ´Ô´Łş ĚâĐÍŁşĘľŃéĚâ
ij͏ѧÓĂ0.10 mol/LľÄHClČÜŇş˛âś¨Î´ÖŞĹ¨śČľÄNaOHČÜŇşŁŹĆäĘľŃé˛Ů×÷ČçĎÂŁş
| AŁŽÓĂËáĘ˝ľÎś¨šÜÁżČĄ20.00 mL HClČÜҺעČëלĐÎĆżŁŹÍŹĘąľÎźÓ2-3ľÎˇÓĚŞĘÔŇşŁť |
| BŁŽÓĂ0.10 mol/LľÄHC!ČÜŇşČóĎ´ËáĘ˝ľÎś¨šÜŁť |
| CŁŽ°ŃľÎś¨šÜÓĂŐôÁóËŽĎ´žťŁť |
| DŁŽČĄĎÂźîĘ˝ľÎś¨šÜŁŹÓĂ´ý˛âNaOHČÜŇşČóĎ´şóŁŹ˝Ť´ý˛âNaOHČÜҺעČëźîĘ˝ľÎś¨šÜÖÁžŕŔëżĚśČĄ°0ĄąŇÔÉĎ20 cm´ŚŁŹÔŮ°ŃźîĘ˝ľÎś¨šÜšĚś¨şĂŁŹľ÷˝ÚŇşĂ棝 |

˛éż´´đ°¸şÍ˝âÎö>>
żĆÄżŁş¸ßÖĐťŻŃ§ Ŕ´Ô´Łş ĚâĐÍŁşĘľŃéĚâ
ľÎś¨ĘľŃéĘÇťŻŃ§Ń§żĆÖĐÖŘŇŞľÄś¨ÁżĘľŃ饣
ÇëťŘ´đĎÂÁĐÎĘĚ⣺
Ł¨1ŁŠËáźîÖк;Μ¨ĄŞĄŞÓĂąęןŃÎËáľÎś¨Î´ÖŞĹ¨śČľÄNaOHČÜŇşĄŁ
˘ŮĎÂÁвŮ×÷ÔěłÉ˛âś¨˝ášűĆŤ¸ßľÄĘÇ (ĚîŃĄĎî×Öĸ)
AŁŽľÎś¨ÖŐľăśÁĘýĘąŁŹ¸ŠĘӾΜ¨šÜżĚśČŁŹĆäËű˛Ů×÷ŐýȡĄŁ
BŁŽĘ˘×°Î´ÖŞŇşľÄלĐÎĆżÓĂŐôÁóËŽĎ´šýŁŹÎ´ÓĂδ֪ҺČóĎ´
CŁŽËáĘ˝ľÎś¨šÜÓĂŐôÁóËŽĎ´žťşóŁŹÎ´ÓĂąęןŃÎËáČóĎ´
DŁŽľÎś¨Ç°ŁŹľÎś¨šÜźâ×ěÓĐĆřĹÝŁŹľÎś¨şóĆřĹÝĎűʧ
˘Ú¸ĂѧÉúľÄĘľŃé˛Ů×÷ČçĎÂŁş
AĄ˘ÓĂźîĘ˝ľÎś¨šÜČĄĎĄNaOH 25.00mLŁŹ×˘ČëלĐÎĆżÖĐŁŹźÓČëź×ťůłČ×öָʞźÁĄŁ
BĄ˘ÓĂ´ý˛âś¨ľÄČÜŇşČóĎ´źîĘ˝ľÎś¨šÜĄŁ
CĄ˘ÓĂŐôÁóËŽĎ´¸ÉžťľÎś¨šÜĄŁ
DĄ˘ČĄĎÂËáĘ˝ľÎś¨šÜÓĂąęןľÄHClČÜŇşČóĎ´şóŁŹ˝ŤąęןҺעČëľÎś¨šÜżĚśČĄ°0ĄąŇÔÉĎ2ĄŤ3cm´ŚŁŹÔٰѾΜ¨šÜšĚś¨şĂŁŹľ÷˝ÚŇşĂćÖÁżĚśČĄ°0ĄąťňĄ°0ĄążĚśČŇÔĎÂĄŁ
EĄ˘źě˛éľÎś¨šÜĘǡńŠˎĄŁ
FĄ˘ÁíȥלĐÎĆżŁŹÔŮÖظ´˛Ů×÷Ňť´ÎĄŁ
GĄ˘°ŃלĐÎĆżˇĹÔھΜ¨šÜĎÂĂ棏ƿĎÂľćŇťŐĹ°×Ö˝ŁŹąßľÎąßŇĄśŻ×śĐÎĆżÖąÖÁľÎś¨Öվ㣏źÇĎ¾Μ¨šÜŇşĂćËůÔڿ̜ȥŁ
a. ľÎś¨˛Ů×÷ľÄŐýȡ˳ĐňĘÇŁ¨ÓĂĐňşĹĚîĐ´ŁŠ___________________________ ĄŁ
b. ÔÚG˛Ů×÷ÖĐČçşÎȡś¨Öվ㣿 __________________________ ____ ĄŁ
c. ČôťťˇÓĚŞ×öָʞźÁŁŹČçşÎȡś¨Öվ㣿 _________________________ ____ ĄŁ
Ł¨2ŁŠŃőťŻťšÔľÎś¨ĄŞČĄ˛ÝËáČÜŇşÖĂÓÚלĐÎĆżÖĐŁŹźÓČëĘĘÁżĎĄÁňËᣏÓĂŨśČÎŞ0.1molĄ¤LŁ1ľÄ¸ßĂĚËáźŘČÜŇşľÎś¨ŁŹˇ˘ÉúľÄˇ´ÓŚÎŞŁş2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2Ąü+2MnSO4+8H2OĄŁąí¸ńÖĐźÇÂźÁËĘľŃéĘýžÝŁş
| ľÎś¨´ÎĘý | ´ý˛âŇşĚĺťý (mL)[Ŕ´ | ąęןKMnO4ČÜŇşĚĺťý(mL) | |
| ľÎś¨Ç°śÁĘý | ľÎś¨şóśÁĘý | ||
| ľÚŇť´Î | 25.00 | 0.50 | 20.40 |
| ľÚśţ´Î | 25.00 | 3.00 | 23.00 |
| ľÚČý´Î | 25.00 | 4.00 | 24.10 |
| ÄŃČÜÎď | AgCl | AgBr | AgCN | Ag2CrO4 | AgSCN |
| ŃŐÉŤ | °× | ÇłťĆ | °× | ׊şě | °× |
| Ksp | 1.77ĄÁ10Ł10 | 5.35ĄÁ10Ł13 | 1.21ĄÁ10Ł16 | 1.12ĄÁ10Ł12 | 1.0ĄÁ10Ł12 |
˛éż´´đ°¸şÍ˝âÎö>>
żĆÄżŁş¸ßÖĐťŻŃ§ Ŕ´Ô´Łş ĚâĐÍŁşĘľŃéĚâ
ijѧÉúÔÚ0.1mol/LNaHCO3ČÜŇşÖоΟӡÓĚŞČÜŇş1ľÎŁŹŐű¸öČÜŇşź¸şőĂťÓĐʲôąäťŻŁŹľŤČÜŇşźÓČČşóŁŹĎÔĂ÷ĎÔľşěÉŤŁŹźÓČȽϳ¤ĘąźäşóŔäČ´ŁŹşěÉŤ˛ťÍĘČĽĄŁ
¸ĂѧÉúÎŞÁËÁË˝â¸ĂšýłĚľÄÔŇňŁŹ˝řĐĐÁËĎÂÁĐĚ˝žżšýłĚŁş
ĄžĘľŃéĚ˝žżĄż
ĘľŃé1: źÓČČ0.1mol/LNaHCO3ČÜŇşŁŹ˛âľĂČÜŇşpHąäťŻČçĎÂąí
| Îœȣ¨ĄćŁŠ | 10 | 20 | 30 | 50 | 70 | 80 | 100 |
| pH | 8.3 | 8.4 | 8.5 | 8.9 | 9.4 | 9.6 | 10.1 |
˛éż´´đ°¸şÍ˝âÎö>>
żĆÄżŁş¸ßÖĐťŻŃ§ Ŕ´Ô´Łş ĚâĐÍŁşľĽŃĄĚâ
ČçÍźËůĘž×°ÖĂÖĐ,a?bśźĘÇśčĐԾ矍,ͨľçŇťśÎĘąźäşó(ľç˝âŇş×ăÁż),aźŤ¸˝˝üČÜŇşĎÔşěÉŤ?ĎÂÁĐËľˇ¨ŐýȡľÄĘÇ
| AŁŽaĘǸşźŤ,bĘÇŐýźŤ |
| BŁŽCuSO4ČÜŇşľÄpHÖ𽼟őĐĄ |
CŁŽÍľçźŤÉϾġ´ÓŚĘ˝ÎŞ |
| DŁŽĎňNaClČÜŇşÖĐźÓČëŇťś¨ÁżľÄŃÎËá,ÄÜĘšČÜŇşÓëÔŔ´ČÜŇşÍęČŤŇťŃů |
˛éż´´đ°¸şÍ˝âÎö>>
żĆÄżŁş¸ßÖĐťŻŃ§ Ŕ´Ô´Łş ĚâĐÍŁşľĽŃĄĚâ
ČźÁĎľçłŘĘÇČźÁĎŁ¨ČçH2Ą˘COĄ˘CH4ľČŁŠ¸úŃőĆřťňżŐĆřĆđˇ´ÓŚŁŹ˝ŤťŻŃ§ÄÜתąäÎŞľçÄÜľÄ×°ÖĂŁŹľç˝âҺΪǿźîČÜŇşĄŁĎÂÁĐšŘÓÚź×ÍéČźÁĎľçłŘľÄËľˇ¨ŐýȡľÄĘÇŁ¨ ŁŠ
| AŁŽ¸şźŤˇ´ÓŚÎŞCH4+10OHŁĄúCO32Ł+7H2O+8eŁ |
| BŁŽ¸şźŤˇ´ÓŚÎŞO2+2H2O+4eŁĄú4OHŁ |
| CŁŽËćךžç˝řĐĐŁŹČÜŇşÖĐľÄŃôŔë×ÓĎň¸şźŤŇĆśŻ |
| DŁŽËćךžç˝řĐĐŁŹČÜŇşľÄPH˛ťąä |
˛éż´´đ°¸şÍ˝âÎö>>
°ŮśČÖÂĐĹ - ÁˇĎ°˛áÁĐąí - ĘÔĚâÁĐąí
şţąąĘĄťĽÁŞÍřÎĽˇ¨şÍ˛ťÁźĐĹϢžŮą¨Ć˝Ě¨ | ÍřÉĎÓĐşŚĐĹϢžŮą¨×¨Çř | ľçĐĹՊƞٹ¨×¨Çř | ÉćŔúʡĐéÎŢÖ÷ŇĺÓĐşŚĐĹϢžŮą¨×¨Çř | ÉćĆóÇÖȨžŮą¨×¨Çř
ÎĽˇ¨şÍ˛ťÁźĐĹϢžŮą¨ľçť°Łş027-86699610 žŮą¨ÓĘĎ䣺58377363@163.com