
·ÖĪö £Ø1£©¢ŁĻŌÓ°Ņŗæɽ«AgBr×Ŗ»ÆĪŖAg£¬ÕāŅ»¹ż³ĢÖŠ£¬Ag“Ó+1”ś0£¬»ÆŗĻ¼Ū½µµĶ£¬±»»¹Ō£¬¾Ż“ĖæÉĶĘ²āĻŌÓ°Ņŗ¾ßÓŠ»¹ŌŠŌ£»
¢Ś¶ØÓ°¹ż³ĢŹĒÓƶØÓ°Ņŗ£Øŗ¬Na2S2O3£©½«½ŗʬÉĻĪ“øŠ¹āµÄAgBr×Ŗ»ÆĪŖAg£ØS2O3£©23-£¬ŠĪ³ÉĶø¹āĮĮÓ°Ēų£¬¾Ż“ĖŠ“³ö·“Ó¦µÄĄė×Ó·½³ĢŹ½£»
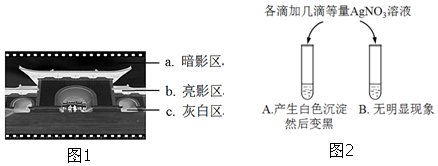
¢Ū“ÓĻŌÓ°¹ż³ĢæÉŅŌ擳ö£¬ÓĆĻŌÓ°Ņŗ½«ĻŌÓ°Ēų±»»ī»ÆµÄAgBr×Ŗ»ÆĪŖAg£¬ŠĪ³É°µÓ°Ēų£¬æÉÅŠ¶Ļ°µÓ°Ēųŗ¬Ag½Ļ¶ą£»
£Ø2£©¢Łøł¾ŻiÖŠ·“Ó¦µÄĘ½ŗā³£ŹżÅŠ¶ĻAgBrµÄĪČ¶ØŠŌŗĶAg£ØNH3£©2+ µÄĪČ¶ØŠŌ£¬¾Ż“Ė½ā“š£»
¢Śa£®ŠĀÖʶØÓ°ŅŗŗĶ·Ļ¶ØÓ°ŅŗµÄ×é³É²»Ķ¬£¬ŠĀÖʶØÓ°ŅŗÖŠ¼ÓČėĻõĖįŅųČÜŅŗĻČŹĒ²śÉśNa3Ag£ØS2O3£©2£¬ĪŽĆ÷ĻŌĻÖĻó£¬Čō¼ĢŠųµĪ¼ÓĻõĖįŅųČÜŅŗ£¬»į“ŁŹ¹·“Ó¦2Ag£ØS2O3£©23-£Øaq£©?Ag2S2O3£Øs£©+3S2O32-£Øaq£©ÕżĻņŅʶÆÉś³É
°×É«Ag2S2O3£¬ÓÖ·Ö½ā³öŗŚÉ«Ag2S£»
b£®AŹŌ¹ÜÖŠ“ęŌŚ»ÆŃ§Ę½ŗā£¬2Ag£ØS2O3£©23-£Øaq£©?Ag2S2O3£Øs£©+3S2O32-£Øaq£©£¬¼ÓČėAg+ÓėS2O32-½įŗĻÉś³É°×É«Ag2S2O3£¬°×É«Ag2S2O3Ņ×·Ö½ā³öŗŚÉ«Ag2S£¬±äĪŖŗŚÉ«£®
½ā“š ½ā£ŗ£Ø1£©¢ŁĻŌÓ°Ņŗæɽ«AgBr×Ŗ»ÆĪŖAg£¬ÕāŅ»¹ż³ĢÖŠ£¬Ag“Ó+1”ś0£¬»ÆŗĻ¼Ū½µµĶ£¬±»»¹Ō£¬AgBrŹĒ±»ĻŌÓ°Ņŗ»¹ŌµÄ£¬Ņņ“ĖĻŌÓ°Ņŗ¾ßÓŠ»¹ŌŠŌ£¬
¹Ź“š°øĪŖ£ŗ»¹Ō£»
¢Śøł¾ŻĢāøÉĆčŹö£¬¶ØÓ°¹ż³ĢŹĒÓƶØÓ°Ņŗ£Øŗ¬Na2S2O3£©½«½ŗʬÉĻĪ“øŠ¹āµÄAgBr×Ŗ»ÆĪŖAg£ØS2O3£©23-£¬ÕāŅ»¹ż³ĢÖŠ£¬AgBrÓėNa2S2O3·“Ӧɜ³ÉÅäŗĻĪļAg£ØS2O3£©23-£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗ2S2O32-+AgBrØTAg£ØS2O3£©23-+Br-£¬
¹Ź“š°øĪŖ£ŗ2S2O32-+AgBrØTAg£ØS2O3£©23-+Br-£»
¢ŪĻŌÓ°¹ż³ĢĪŖ£ŗÓĆĻŌÓ°Ņŗ½«ĻŌÓ°Ēų±»»ī»ÆµÄAgBr×Ŗ»ÆĪŖAg£¬ŠĪ³É°µÓ°Ēų£¬Ņņ“ĖĶ¼1ÖŠµÄŅ»Õž³åÓ”ŗóµĆµ½µÄŗŚ°×µ×ʬ֊£¬ĘäÖŠŗ¬ŅųŌŖĖŲ×ī¶ąµÄĒųÓņŹĒaĒųÓņ£¬
¹Ź“š°øĪŖ£ŗa£»
£Ø2£©¢ŁŅŃÖŖ£ŗAg+£Øaq£©+Br-£Øaq£©?AgBr£Øs£© K1=1.9”Į1012£¬
Ag+£Øaq£©+2NH3•H2O£Øaq£©?Ag£ØNH3£©2+ £Øaq£©+2H2O£Øl£© K2=1.1”Į107£¬
ĻŌČ»K1£¾K2£¬±ķĆ÷AgBrµÄĪČ¶ØŠŌ“óÓŚAg£ØNH3£©2+£¬ĖłŅŌ²»ÄÜÓĆ°±Ė®ČÜŅŗĢę“ś£¬ĄķÓÉŹĒNH3ÓėAg+µÄ½įŗĻÄÜĮ¦ČõÓŚBr-ÓėAg+µÄ½įŗĻÄÜĮ¦£¬
¹Ź“š°øĪŖ£ŗ²»ÄÜ£»ÓÉK1£¾K2æÉÖŖNH3ÓėAg+µÄ½įŗĻÄÜĮ¦ČõÓŚBr-ÓėAg+µÄ½įŗĻÄÜĮ¦£»
¢Śa£®ŠĀÖʶØÓ°Ņŗŗ¬ÓŠNa2S2O3£¬·Ļ¶ØÓ°Ņŗŗ¬ÓŠNa3Ag£ØS2O3£©2ŗĶÉŁĮæNa2S2O3£¬·Ö±š¼ÓČėĻõĖįŅųČÜŅŗ£¬¶ŌÓŚŠĀÖʶØÓ°Ņŗ£¬·“Ó¦ŹĒ²śÉśNa3Ag£ØS2O3£©2£¬ĪŽĆ÷ĻŌĻÖĻó£¬Ņņ“ĖŹ¢×°ŠĀÖʶØÓ°ŅŗµÄŹŌ¹ÜĪŖB£¬Čō¼ĢŠųµĪ¼ÓĻõĖįŅųČÜŅŗ£¬»įŹ¹Ag£ØS2O3£©23-µÄĮæŌö¶ą£¬“ŁŹ¹»Æѧ·“Ó¦2Ag£ØS2O3£©23-£Øaq£©?Ag2S2O3£Øs£©+3S2O32-£Øaq£©ĻņÓŅŅĘ¶Æ£¬²śÉśAg2S2O3£Øs£©£¬²śÉś°×É«³ĮµķAg2S2O3£Øs£©£¬Č»ŗóAg2S2O3£Øs£©·Ö½āÉś³ÉŗŚÉ«µÄAg2S£¬Ņņ“ĖĻÖĻóĪŖĖęµĪ¼ÓAgNO3µÄĮæµÄŌö¼Ó£¬ČÜŅŗÖŠ²śÉś°×É«³Įµķ£¬Č»ŗó±äŗŚ£»
¹Ź“š°øĪŖ£ŗB£»ĖęµĪ¼ÓAgNO3µÄĮæµÄŌö¼Ó£¬ČÜŅŗÖŠ²śÉś°×É«³Įµķ£¬Č»ŗó±äŗŚ£»
b£®AŹŌ¹ÜÖŠ“ęŌŚ»ÆŃ§Ę½ŗā£¬2Ag£ØS2O3£©23-£Øaq£©?Ag2S2O3£Øs£©+3S2O32-£Øaq£©£¬¼ÓČėAg+ÓėS2O32-½įŗĻÉś
°×É«Ag2S2O3£¬°×É«Ag2S2O3Ņ×·Ö½ā³öŗŚÉ«Ag2S£¬±äĪŖŗŚÉ«£¬Ņņ“ĖŌŅņĪŖ£ŗAŹŌ¹ÜČÜŅŗÖŠ“ęŌŚĘ½ŗā£ŗ2Ag£ØS2O3£©23-£Øaq£©?Ag2S2O3£Øs£©+3S2O32-£Øaq£©£¬µĪČėµÄAg+ÓėS2O32-½įŗĻÉś³ÉAg2S2O3£¬Ź¹c£ØS2O32-£©ĻĀ½µ£¬“Ł½ųÉĻŹöĘ½ŗāÕżĻņŅĘ¶Æ£¬µ¼ÖĀ°×É«Ag2S2O3³Įµķ“óĮæĪö³ö£¬Ag2S2O3ÓÖ·Ö½ā³öŗŚÉ«Ag2Sµ¼ÖĀ³Įµķ±äŗŚ£¬
¹Ź“š°øĪŖ£ŗAŹŌ¹ÜČÜŅŗÖŠ“ęŌŚĘ½ŗā£ŗ2Ag£ØS2O3£©23-£Øaq£©?Ag2S2O3£Øs£©+3S2O32-£Øaq£©£¬µĪČėµÄAg+ÓėS2O32-½įŗĻÉś³ÉAg2S2O3£¬Ź¹c£ØS2O32-£©ĻĀ½µ£¬“Ł½ųÉĻŹöĘ½ŗāÕżĻņŅĘ¶Æ£¬µ¼ÖĀ°×É«Ag2S2O3³Įµķ“óĮæĪö³ö£¬Ag2S2O3ÓÖ·Ö½ā³öŗŚÉ«Ag2Sµ¼ÖĀ³Įµķ±äŗŚ£®
µćĘĄ ±¾Ģāæ¼²é³ĮµķµÄČܽāĘ½ŗā£¬ÅäŗĻĪļµÄÉś³É£¬»ÆŃ§Ę½ŗāµÄŅĘ¶Æ£¬ĢāÄææ¼²é×ŪŗĻ£¬Šč½įŗĻ·“Ó¦µÄ·½³ĢŹ½×ŠĻø·ÖĪö£¬ĪŖŅדķĢā£®ĢāÄæÄŃ¶Č½Ļ“ó£¬ŹĒÄŃĢā£®


 ŹĄ¼Ķ°ŁĶØĘŚÄ©½š¾ķĻµĮŠ“š°ø
ŹĄ¼Ķ°ŁĶØĘŚÄ©½š¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¼×ĶéŗĶ±½¶¼Ć»ÓŠĶ¬·ÖŅģ¹¹Ģå | |
| B£® | ¼×±½£Ø £©µÄ·Ö×ÓÖŠĖłÓŠĢ¼Ō×ÓŌŚĶ¬Ņ»Ę½ĆęÉĻ £©µÄ·Ö×ÓÖŠĖłÓŠĢ¼Ō×ÓŌŚĶ¬Ņ»Ę½ĆęÉĻ | |
| C£® | ±½ŗĶŅŅĻ©¶¼ÄÜŹ¹äåĖ®ĶŹÉ«£¬¾łÓėäåĖ®·¢Éś¼Ó³É·“Ó¦ | |
| D£® | ŅŅĻ©ŌŚ¹¤ŅµÉĻÖ÷ŅŖĶعżŹÆÓĶ·ÖĮóµĆµ½ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
 ¼×“¼ŹĒŅ»ÖÖÓÅÖŹČ¼ĮĻ£¬ŌŚ¹¤ŅµÉĻ³£ÓĆ
¼×“¼ŹĒŅ»ÖÖÓÅÖŹČ¼ĮĻ£¬ŌŚ¹¤ŅµÉĻ³£ÓĆ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| ŹµŃé²Ł×÷ | ŹµŃéÄæµÄ | |
| A | ½«SO2ĶØČė×ĻÉ«ŹÆČļČÜŅŗÖŠ | Ö¤Ć÷SO2¾ßÓŠĖįŠŌŗĶĘư׊Ō |
| B | ÓĆpHŹŌÖ½²ā¶ØNaHSO3ČÜŅŗµÄpH | ±Č½ĻHSO3-µēĄė³Ģ¶ČŗĶĖ®½ā³Ģ¶ČµÄ“óŠ” |
| C | äåŅŅĶéÓėNaOHŅŅ“¼ČÜŅŗ¹²ČČ£¬½«µĆµ½µÄĘųĢåĶØČėäåµÄĖÄĀČ»ÆĢ¼ČÜŅŗ | Ö¤Ć÷·“Ó¦µĆµ½µÄĘųĢåŗ¬ÓŠŅŅĻ© |
| D | Ļņŗ¬·ÓĢŖµÄNa2SO3ČÜŅŗÖŠ¼ÓČėBaC12ČÜŅŗ | Ö¤Ć÷Na2SO3ČÜŅŗÖŠ“ęŌŚĖ®½āĘ½ŗā |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÅØ¶Č¾łĪŖ2”Į10-5mol•L-1µÄAgNO3ČÜŅŗŗĶNaClČÜŅŗµČĢå»ż»ģŗĻ£¬ÓŠ°×É«³Įµķ²śÉś | |
| B£® | ½«0.001mol•L-1µÄAgNO3ČÜŅŗµĪČė0.001mol•L-1µÄKClŗĶK2CrO4µÄ»ģŗĻČÜŅŗÖŠ£¬ĻČ²śÉśAgCl³Įµķ | |
| C£® | c£ØMg2+£©ĪŖ0.11mol•L-1µÄČÜŅŗÖŠŅŖ²śÉśMg£ØOH£©2³Įµķ£¬ČÜŅŗµÄpHŅŖæŲÖĘŌŚ9ŅŌÉĻ | |
| D£® | ĘäĖūĢõ¼ž²»±ä£¬Ļņ±„ŗĶAg2CrO4Ė®ČÜŅŗÖŠ¼ÓČėÉŁĮæK2CrO4ČÜŅŗ£¬ČÜŅŗÖŠc£ØAg+£©¼õŠ” |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¢Ł¢Ś¢Ū¢Ü¢Ż | B£® | ¢Ł¢Ū¢Ž | C£® | ¢Ü¢Ż¢ß | D£® | ¢Ś¢Ü¢Ž |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
 ŌŚ¹ĢĢ¬½šŹōŃõ»ÆĪļµē½ā³ŲÖŠ£¬øßĪĀ¹²µē½āH2O-CO2»ģŗĻĘųĢåÖʱøH2ŗĶCOŹĒŅ»ÖÖŠĀµÄÄÜŌ“ĄūÓĆ·½Ź½£¬»ł±¾ŌĄķČēĶ¼ĖłŹ¾£®Ņõ¼«µÄ·“Ó¦Ź½ŹĒ£ŗH2O+2e-ØTH2”ü+O2-£¬CO2+2e-ØTCO+O2-£»Ńō¼«µÄ·“Ó¦Ź½ŹĒ£ŗ2H2O-4e-ØTO2”ü+4H+£®×Ü·“Ó¦æɱķŹ¾ĪŖ£ŗH2O+CO2$\frac{\underline{\;µē½ā\;}}{\;}$H2+CO+O2£®
ŌŚ¹ĢĢ¬½šŹōŃõ»ÆĪļµē½ā³ŲÖŠ£¬øßĪĀ¹²µē½āH2O-CO2»ģŗĻĘųĢåÖʱøH2ŗĶCOŹĒŅ»ÖÖŠĀµÄÄÜŌ“ĄūÓĆ·½Ź½£¬»ł±¾ŌĄķČēĶ¼ĖłŹ¾£®Ņõ¼«µÄ·“Ó¦Ź½ŹĒ£ŗH2O+2e-ØTH2”ü+O2-£¬CO2+2e-ØTCO+O2-£»Ńō¼«µÄ·“Ó¦Ź½ŹĒ£ŗ2H2O-4e-ØTO2”ü+4H+£®×Ü·“Ó¦æɱķŹ¾ĪŖ£ŗH2O+CO2$\frac{\underline{\;µē½ā\;}}{\;}$H2+CO+O2£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ×å ÖÜĘŚ | IA | IIA | IIIA | IVA | VA | VIA | VIIA |
| ¶ž | ] | ¢Ł | ¢Ś | ||||
| Čż | ¢Ū | ¢Ü | ¢Ż | ¢Ž | |||
| ĖÄ | ¢ß |
 £®
£® £¬½«øĆĒā»ÆĪļĶØČė¢ÜŗĶ¢ŽŠĪ³ÉµÄ»ÆŗĻĪļµÄĖ®ČÜŅŗÖŠ£¬·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖAl3++3NH3•H2O=Al£ØOH£©3”ż+3NH4+£®
£¬½«øĆĒā»ÆĪļĶØČė¢ÜŗĶ¢ŽŠĪ³ÉµÄ»ÆŗĻĪļµÄĖ®ČÜŅŗÖŠ£¬·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖAl3++3NH3•H2O=Al£ØOH£©3”ż+3NH4+£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | dµÄ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦Ė®»ÆĪļæÉČÜÓŚcµÄ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦µÄĖ®»ÆĪļµÄČÜŅŗ | |
| B£® | aŗĶcŠĪ³ÉµÄĄė×Ó»ÆŗĻĪļÖŠæÉÄÜŗ¬ÓŠ¹²¼Ū¼ü | |
| C£® | ĖÄÖÖŌŖĖŲĄė×Ó°ė¾¶£ŗc£¾d£¾a£¾b | |
| D£® | cŹĒ¶ĢÖÜĘŚŌŖĖŲÖŠ½šŹōŠŌ×īĒæµÄŌŖĖŲ£¬bŹĒ¶ĢÖÜĘŚŌŖĖŲÖŠ·Ē½šŹōŠŌ×īĒæµÄŌŖĖŲ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com